Intracellular Ca2+ Release Channels: RyRs and IP3Rs
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
67 Terms
What are the two types of intracellular Ca2+ release channels discussed in the notes?
Ryanodine Receptors (RyR) and IP3 Receptors (IP3R)
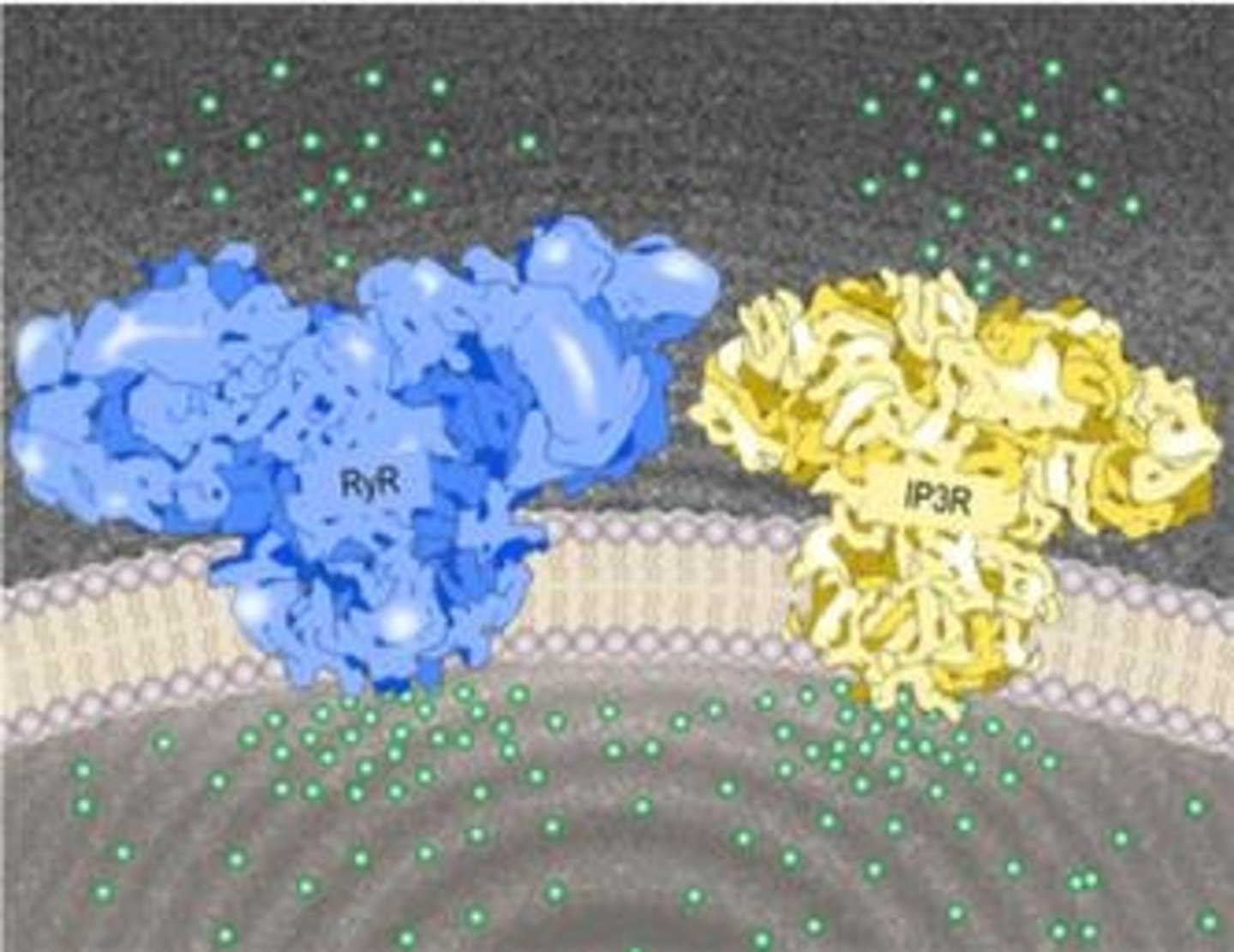
What is the primary function of the Endoplasmic Reticulum (ER)?
Synthesis and storage of proteins and fats, and transportation conduits for cellular materials.
How does Ca2+ function in relation to IP3Rs?
Ca2+ is the main activator of IP3Rs, increasing the probability of their opening at concentrations slightly above cytoplasmic levels (~50-100 nM).
What happens to IP3R activity at higher cytoplasmic Ca2+ concentrations?
At higher concentrations (above 2-3 µM), Ca2+ inhibits IP3R opening, preventing excessive Ca2+ release.
What role does IP3 play in the activation of IP3Rs?
IP3 disables the feedback inhibition of Ca2+, allowing IP3Rs to remain open and release Ca2+ from stores.
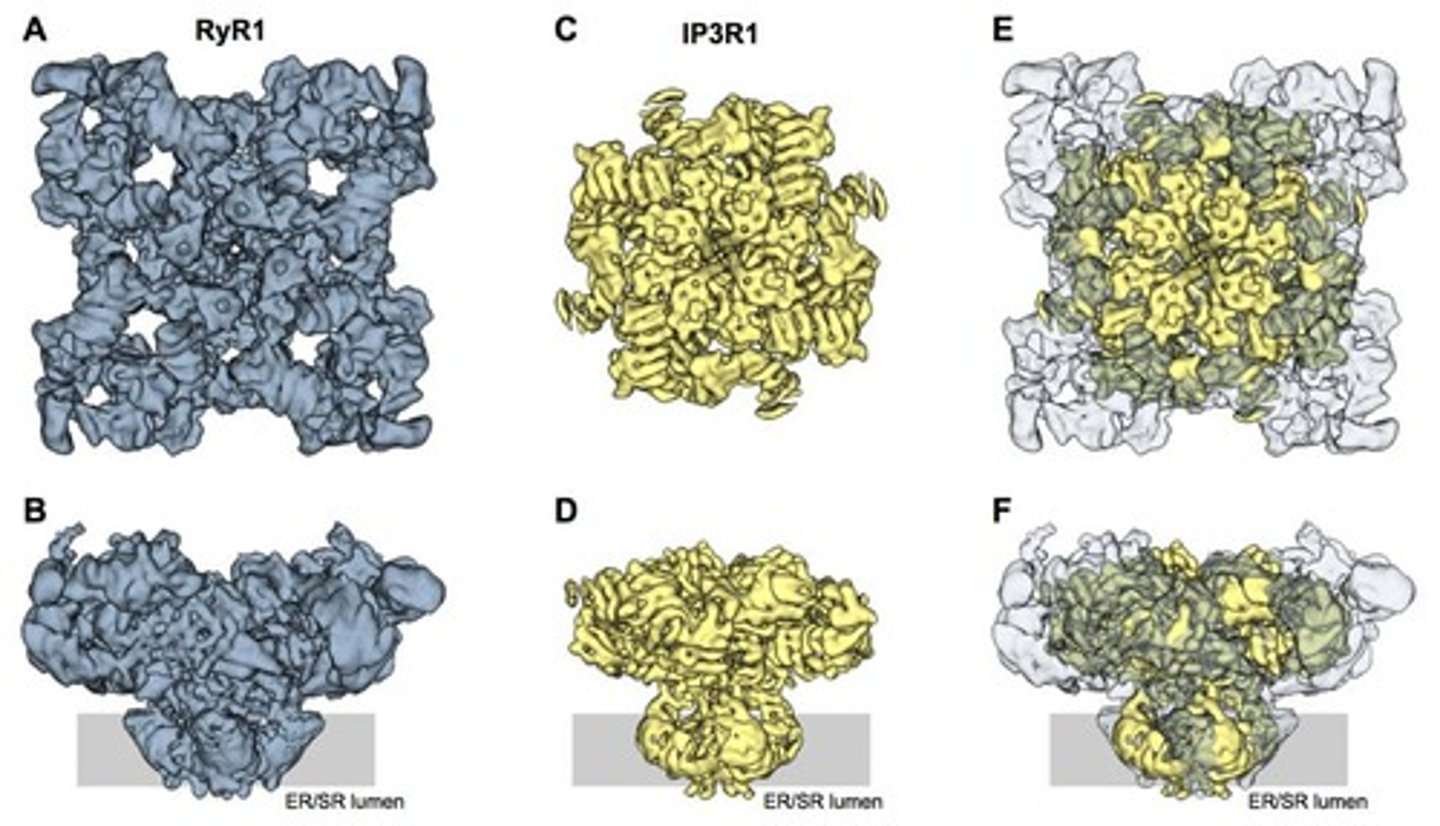
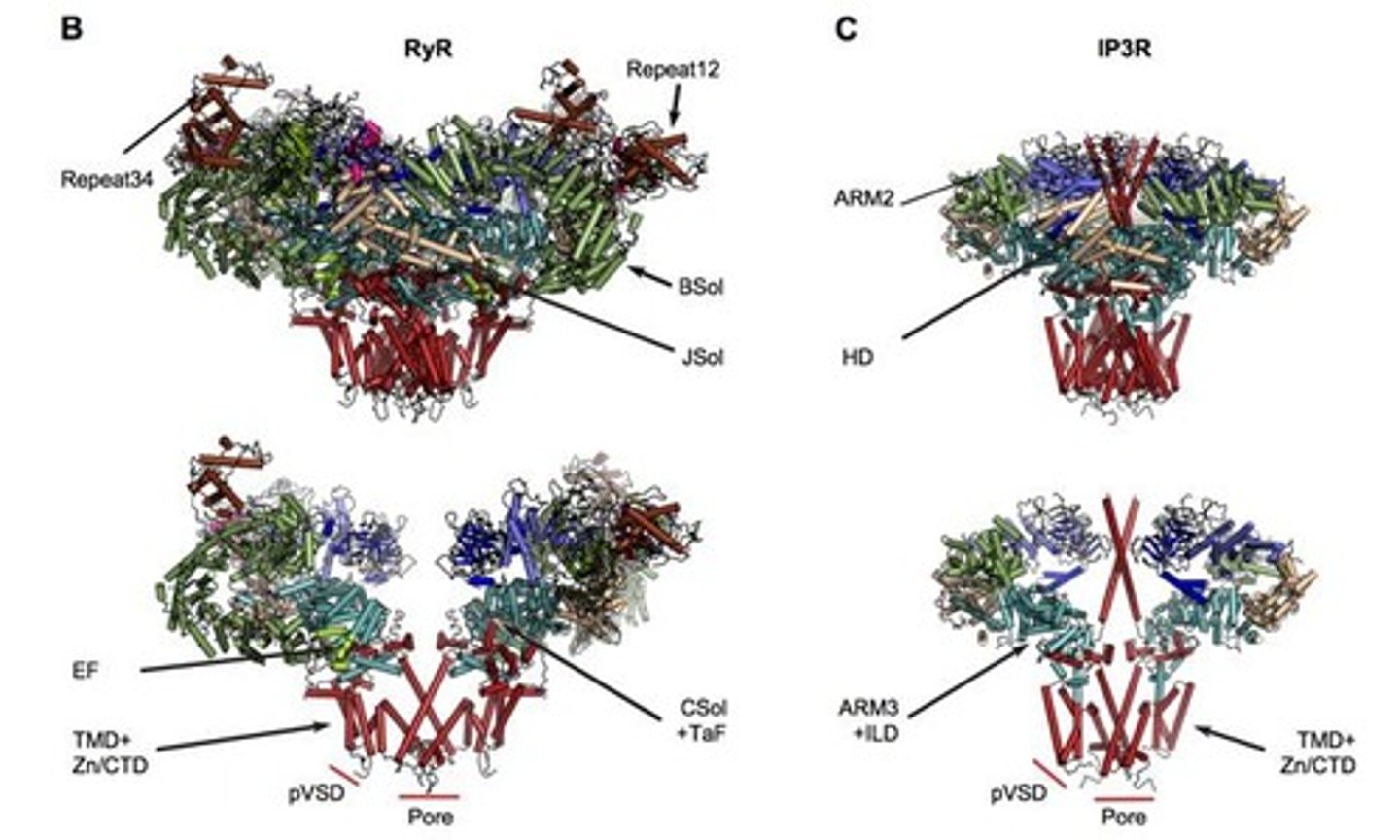
What is the structural composition of an IP3 receptor?
Each IP3 receptor subunit has 6 transmembrane domains and the functional channel is a tetramer, approximately 1,000 kD in size.
What are the three subtypes of IP3 receptors and where are they predominantly expressed?
IP3R1 is highly expressed in neurons, IP3R2 in hepatocytes (liver), and IP3R3 in rapidly proliferating cells like epithelial cells.
What is the significance of the Ca2+ binding sites on the IP3 receptor?
There are 2 Ca2+ binding sites on each subunit, which are crucial for channel opening and function.
How does IP3 affect the feedback inhibition of IP3Rs?
IP3 allows IP3Rs to remain open by disabling the feedback inhibition normally exerted by higher levels of Ca2+.
What is the physiological role of Ca2+ signaling in cells?
Ca2+ signaling is essential for various cellular processes, including muscle contraction, neurotransmitter release, and gene expression.
What is the effect of ATP on IP3 receptors?
ATP acts as an activator of IP3 receptors.
How does the structure of IP3Rs contribute to their function?
The structural domains of IP3Rs, including the N-terminal domain and luminal loops, facilitate conformational changes necessary for channel opening.
What is the role of GqPCRs in relation to IP3Rs?
GqPCRs are involved in the activation of IP3 production, which in turn activates IP3Rs.
What is the consequence of Ca2+ concentrations reaching around 500 nM for IP3R activity?
At around 500 nM, the probability of IP3R opening reaches a maximum.
What is the significance of the negative feedback mechanism in Ca2+ signaling?
It prevents excessive Ca2+ release, maintaining cellular homeostasis.
What are the main activators and inhibitors of IP3Rs?
Activators include IP3 and ATP, while Ca2+ acts as both an activator at low concentrations and an inhibitor at high concentrations.
What is the approximate size of an IP3 receptor channel compared to a RyR?
IP3 receptors are about half the size of RyRs, with a functional channel size of ~1,000 kD.
What is the role of PIP2 in relation to IP3Rs?
PIP2 is a ligand that is involved in the activation of IP3Rs.
What type of feedback mechanism is described in the context of Ca2+ signaling?
A negative feedback mechanism that prevents excessive release of Ca2+ from the ER.
What is the impact of IP3 on the activation rates of IP3Rs?
IP3 does not change the activation rates of IP3Rs in response to lower Ca2+ levels; it primarily disables feedback inhibition.
What are the key structural features of IP3 receptors that allow for their function?
Key features include transmembrane domains, armadillo repeat domains, and large movements of the N-terminal domains during channel opening.
How do IP3R subtypes differ in terms of regulation?
IP3R subtypes are differentially regulated by phosphorylation and binding proteins, affecting their sensitivity to IP3, Ca2+, and ATP.
What is the consequence of IP3Rs remaining open due to IP3's action?
It allows for the accumulation of much higher cytoplasmic Ca2+ concentrations, up to 2 magnitudes greater.
What effect does ATP have on the Ca2+ activation curve of IP3Rs?
ATP shifts the Ca2+ activation curve to the left, making IP3 channels more sensitive and resulting in a higher open probability of IP3R channels at a given Ca2+/IP3 level.
What is the role of CaBP1 in relation to IP3R function?
CaBP1 inhibits IP3R function.
Name three inhibitors of IP3R.
Xestospongin C, 2-aminoethoxydiphenyl borate (2-APB), and Heparin (dextran sulfate).
Which IP3R subtype has the highest binding affinity for IP3?
Type 1 IP3R has the highest binding affinity for IP3, followed by type 2 and type 3.
How does IP3 binding relate to IP3 sensitivity?
IP3 binding does not necessarily reflect IP3 sensitivity; modulation by cellular factors such as ATP, phosphorylation, and binding proteins significantly alters sensitivity.
What is the least abundant phospholipid involved in the synthesis of IP3?
PI(4,5)P2 (PIP2) is the least abundant phospholipid, comprising less than 1% of total phospholipids.
Which receptors activate PLC-β to produce IP3?
Gq-coupled GPCRs activate PLC-β, which cleaves IP3 off PIP2.
What is the role of receptor tyrosine kinases (RTKs) in IP3 production?
RTKs activate PLC-γ to split PIP2 into IP3 and DAG.
How is the IP3 signal turned off?
Adding an additional phosphate to the IP3 molecule renders it biologically inactive.
What regulates IP3-3-kinase activity?
IP3-3-kinase is regulated by CaM (calmodulin) and CaMKII (Ca2+/CaM dependent kinase), with elevated Ca2+ activating these enzymes.
What types of ligands can activate and/or modulate IP3R activity?
Ligands that signal through GqPCRs, such as Acetyl Choline, Cholecystokinin, Thrombin, and Glutamate, as well as growth factors, cytokines, and hormones from RTKs.
What is the significance of spatio-temporal Ca2+ release patterns?
Ca2+ release events from ER/SR stores must overwhelm reuptake and clearance mechanisms to diffuse and interact with neighboring channels.
What happens to cytoplasmic Ca2+ concentration during rapid temporal Ca2+ oscillations?
If Ca2+ release occurs fast enough, clearance mechanisms may not fully clear cytoplasmic Ca2+, leading to elevated baseline Ca2+ levels.
How does the frequency of Ca2+ release affect the magnitude of subsequent events?
Slower frequency events allow for greater magnitude releases because clearance mechanisms have longer to recharge stores.
What is the effect of the prostanoid GqPCR, EP1, on pacemaker activity?
EP1 activation by prostaglandins has a chronotropic effect that drives contractions in the gut wall.
What occurs when the frequency of pacemaker waves exceeds the clearance capacity?
An alternating pattern of Ca2+ release events occurs, where the amplitude of every second wave is smaller, allowing for better recharging of stores.
What is the relationship between IP3 concentration and the duration of Ca2+ release events?
Higher concentrations of IP3 can increase the duration of opening, leading to larger, sustained Ca2+ release events.
What are the implications of Ca2+ signaling on cellular processes?
Ca2+ signaling is crucial for various cellular processes, including muscle contraction, neurotransmitter release, and gene expression.
How do cellular factors influence IP3R sensitivity?
Cellular factors such as ATP, phosphorylation, and binding proteins can significantly alter the sensitivity of IP3R to IP3.
What is the primary function of IP3 in cellular signaling?
IP3 primarily functions to release Ca2+ from intracellular stores, thereby regulating various cellular activities.
What is the role of DAG in relation to IP3?
DAG is left behind when IP3 is cleaved off PIP2 and plays a role in activating protein kinase C (PKC) pathways.
What is the importance of understanding Ca2+ signaling in pharmacology?
Understanding Ca2+ signaling pathways can aid in the development of drugs targeting various diseases linked to Ca2+ dysregulation.
What experimental evidence supports the modulation of IP3R by ATP?
Research by Mak et al. (2001) demonstrates that ATP increases the sensitivity of IP3 channels, shifting the activation curve.
What triggers the activation of other channels in a cluster during Ca2+ signaling?
The increase in the number and duration of small Ca2+ events (blips) in response to IP3.
What is the result of sufficient Ca2+ release from a cluster in Ca2+ signaling?
It can diffuse to the next cluster, producing a regenerating Ca2+ wave.
How does IP3 influence local Ca2+ events?
IP3 can amplify local Ca2+ events, causing them to 'snowball' and produce global increases in intracellular calcium concentration ([Ca2+]i).
What is required for embryonic development in relation to Ca2+ signaling?
IP3R-mediated Ca2+ release is required for embryonic development.
What factors determine whether Ca2+ blips/sparks summate to form a Ca2+ wave?
The number of Ca2+ blips/sparks, synchrony of their activation, ability to outpace local reuptake and Ca2+ efflux mechanisms, and degree of channel modulation by IP3 concentration.
What is the role of Ca2+ events in endothelial cells?
They activate Nitric Oxide Synthase, which produces NO that relaxes overlying smooth muscle.
What is the effect of Carbachol on Ca2+ signaling in vascular endothelium?
Carbachol activates M1 muscarinic receptors, leading to increased Ca2+ prevalence.
What type of disorders are generally caused by mutations in IP3 receptors (IP3Rs)?
Most are gain-of-function disorders, leading to overactive channel activity.
What is Spinocerebellar ataxia associated with?
IP3R1 mutations in neural tissue, causing motor defects due to degeneration of Purkinje cells.
What cognitive impairment is associated with Gillespie syndrome?
It can cause cognitive impairment and sometimes loss of iris.
What condition is associated with IP3R2 mutations?
Anhidrosis, which is the inability to perspire.
What types of cancer are linked to abnormal functioning of IP3Rs?
Cancer types such as squamous cell carcinomas and colorectal cancer, due to Ca2+ involvement in proliferation, differentiation, and migration.
What neurodegenerative diseases are associated with abnormal functioning of IP3Rs?
Huntington's Disease and Familial Alzheimer's Disease.
What causes Huntington's disease?
It is caused by polyglutamine (polyQ) expansion on the N terminus of the cytosolic Huntingtin protein (Htt).
How does polyQ-Htt affect IP3R1 in neurons?
It increases IP3R1 mediated Ca2+ release in response to metabotropic glutamate receptor stimulation.
What is the effect of the PS1 mutant associated with Alzheimer's Disease on Ca2+ release?
It increases Ca2+ release through IP3R in response to IP3, indicating a gain of function.
What does enhanced Ca2+ release from the ER stimulate in Alzheimer's Disease pathology?
It stimulates amyloid beta processing.
How does IP3 affect feedback inhibition of Ca2+ on IP3 receptors?
IP3 disables feedback inhibition, allowing greater amounts of Ca2+ to be effluxed from intracellular stores.
What allows Ca2+ release to be regulated by a larger set of extracellular molecules?
The generation of IP3 via activation of GqPCRs.
What is the significance of the balance of Ca2+ release and clearance/uptake mechanisms?
It allows for the generation of many different spatio-temporal Ca2+ patterns.
What is the general outcome of diseases involving IP3R mutations?
They mostly cause exaggerated Ca2+ release in response to stimuli.