Immunology: Tolerance, Autoimmunity, and Immune Privilege
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
What is immunologic tolerance?
Mechanisms that prevent an immune response from being mounted against the host's own tissues.
What are the two types of immunologic tolerance?
Central tolerance and peripheral tolerance.
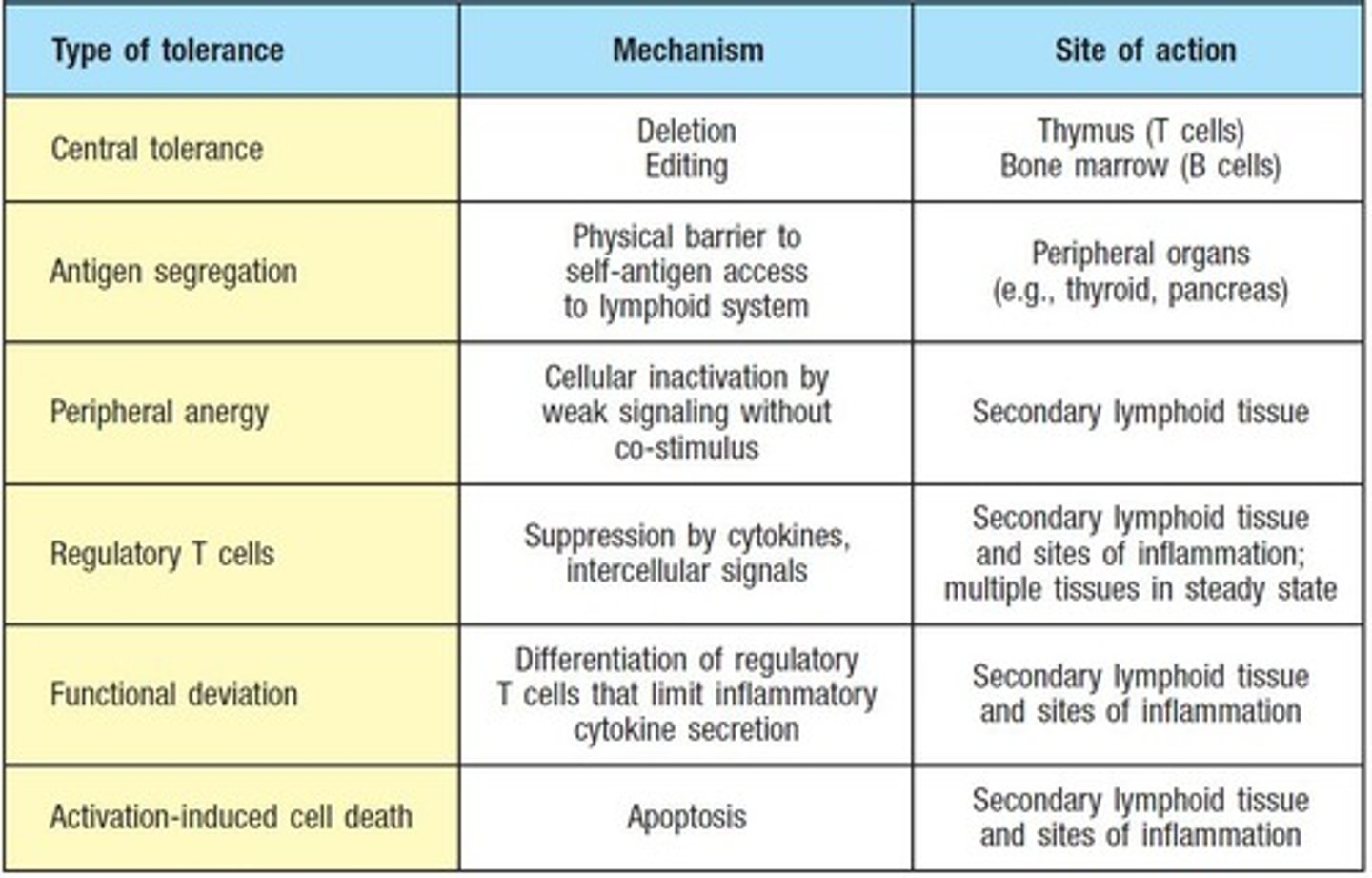
Where does central tolerance occur?
In primary lymphoid organs.
Where does peripheral tolerance occur?
In secondary lymphoid organs, such as lymph nodes and the spleen.
What is autoimmunity?
The loss of the body's tolerance leading to an immune response against its own tissues.
What is the role of the autoimmune regulator (AIRE) protein?
It regulates the expression of tissue-restricted antigens in medullary thymic epithelial cells, leading to the deletion of self-reactive T cells.
What happens during positive selection of thymocytes?
Thymocytes that can interact with self-MHC are selected for survival, while those with very strong or weak binding are eliminated.
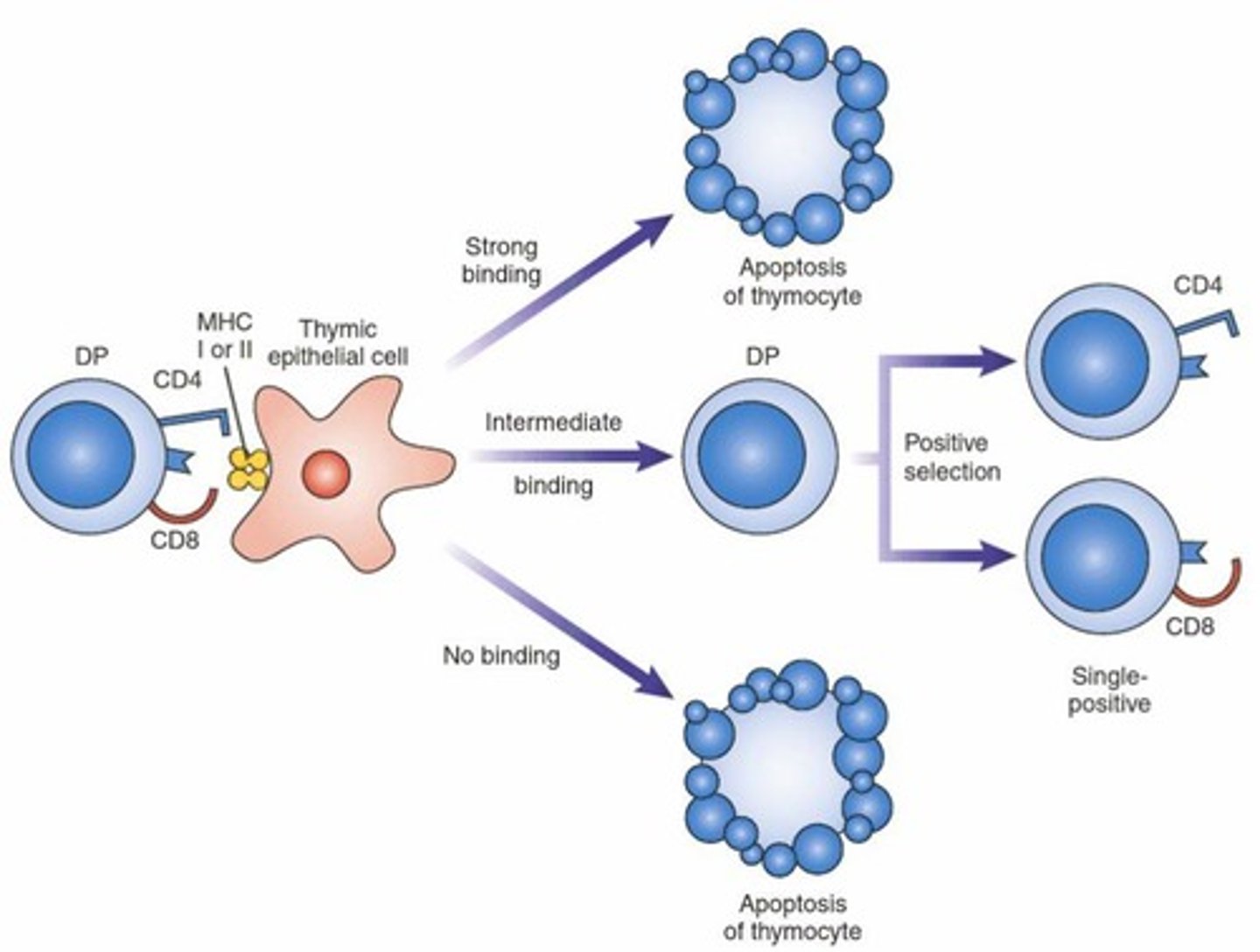
What is negative selection in T-cell development?
The elimination of thymocytes that recognize self-antigens presented by antigen-presenting cells, preventing autoimmunity.
What is anergy in T cells?
A state of functional unresponsiveness that occurs when T cells recognize an antigen without costimulation.
How does CTLA-4 function in T cell regulation?
CTLA-4 inhibits T cell activation by competing with CD28 for binding to B7 ligands and can also be expressed by Tregs to suppress other T cells.
What are Tregs and their role?
Regulatory T cells (CD4+CD25+FoxP3+) suppress immune responses through direct contact, secretion of inhibitory cytokines, and consumption of IL-2.
What is receptor editing in B cells?
A process where immature B cells that recognize self antigens modify their antigen receptors to avoid autoimmunity.
What occurs if an immature B cell recognizes self antigens with high avidity?
The B cell undergoes apoptosis or receptor editing.
What is the significance of weak recognition of soluble self antigens in B cells?
It may lead to anergy, where the B cell becomes functionally inactive.
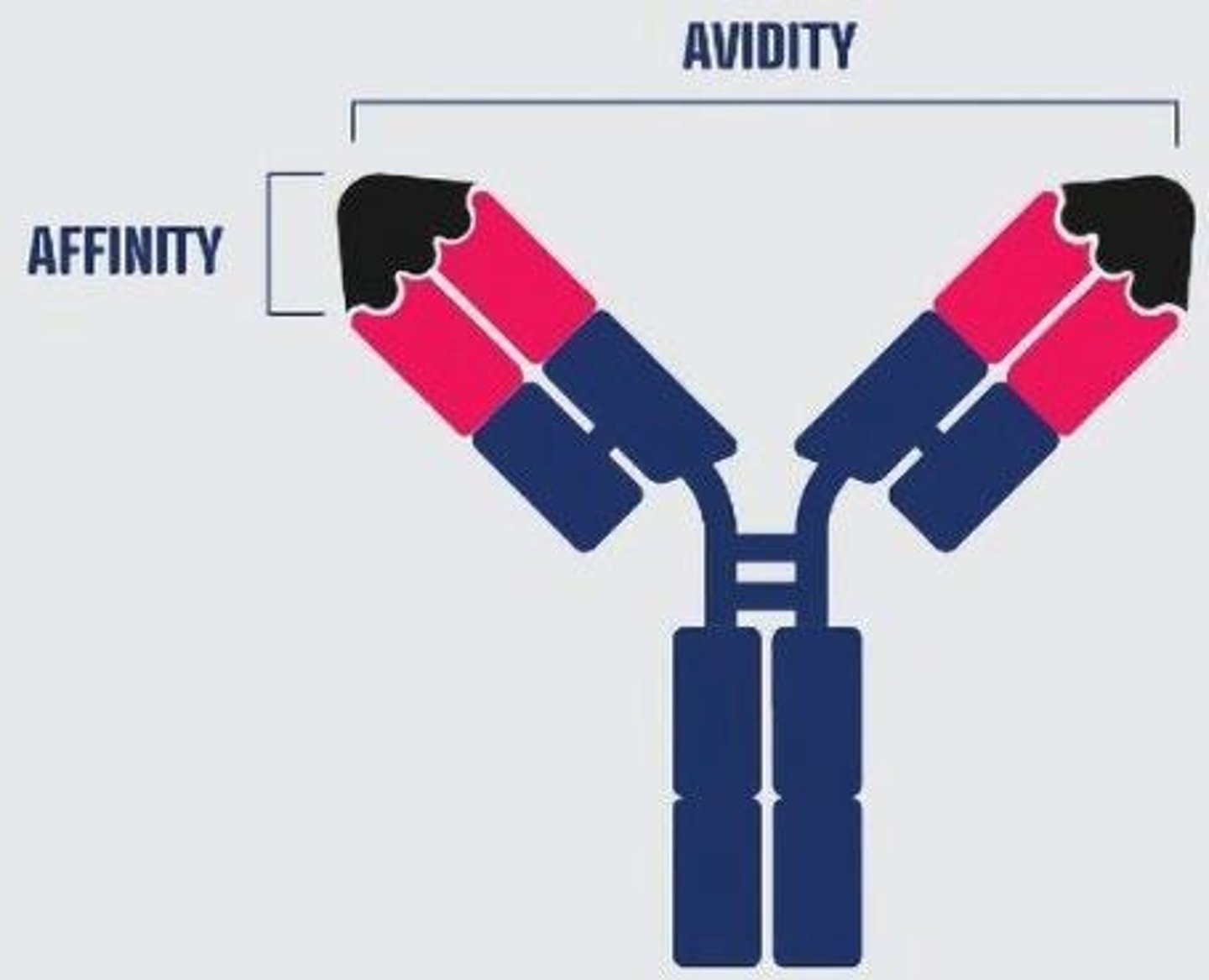
What is the difference between affinity and avidity?
Affinity refers to the strength of binding at a single antigen-binding site, while avidity refers to the total binding strength of a molecule with multiple binding sites.
What are PAMPs?
Pathogen-associated molecular patterns recognized by the immune system.
What are PRRs?
Pattern recognition receptors that detect PAMPs in the immune system.
What is the role of MBL in the immune system?
Mannose-binding lectin (MBL) is involved in the recognition of pathogens and activation of the complement system.
What is the outcome of T cell recognition of self antigens in the thymus?
It leads to the deletion of self-reactive T cells or the development of regulatory T cells.
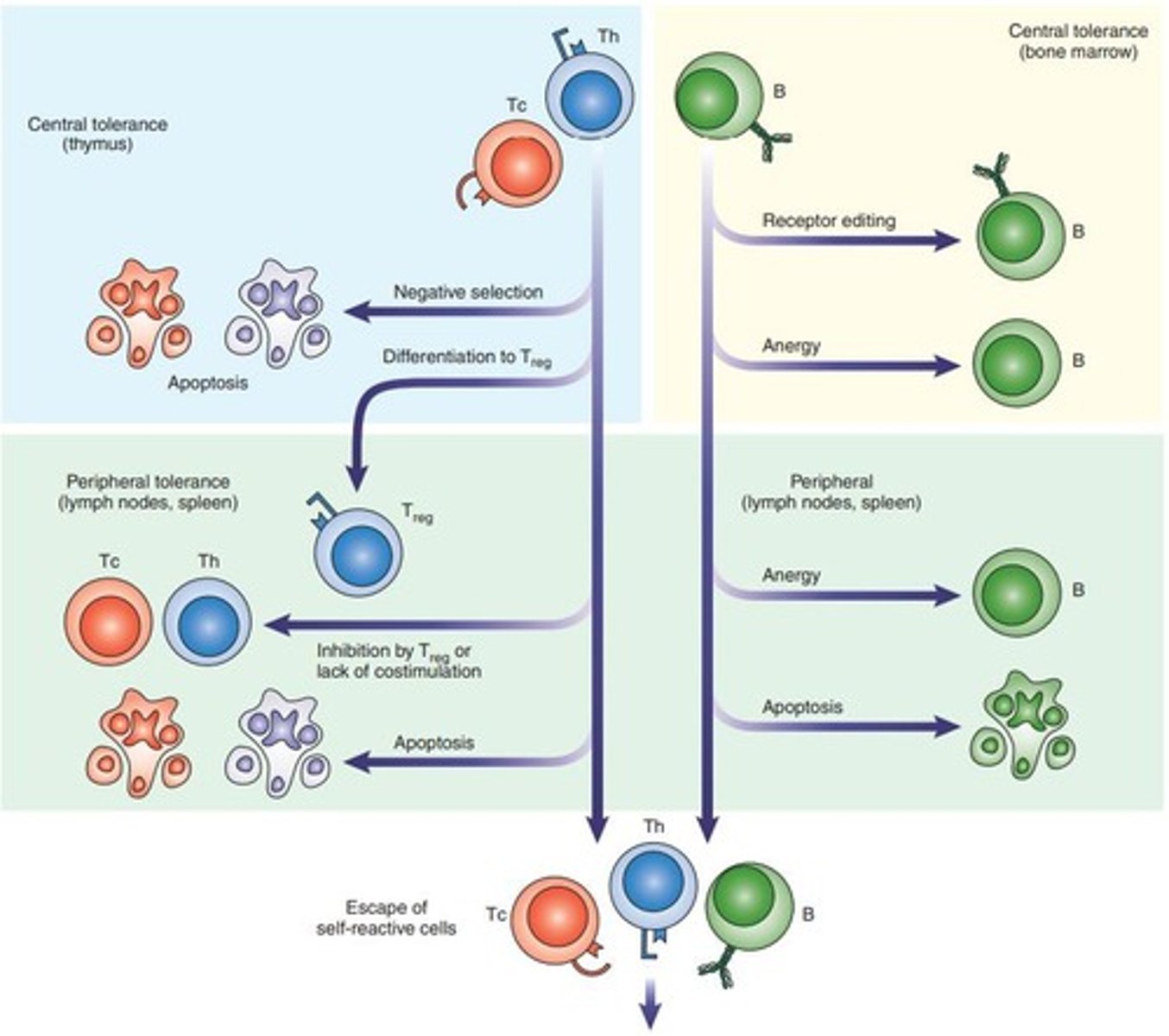
What happens to T cells that recognize self antigens with high affinity in the absence of costimulation?
They may undergo apoptosis.
What is the clinical significance of mutations in the AIRE gene?
They can lead to autoimmune polyendocrine syndrome type 1 (APS1), a multiorgan autoimmune disease.
What is the role of inhibitory receptors like PD-1 in T cell regulation?
They help to downregulate T cell responses and maintain tolerance.
What is the importance of TGF-beta in Treg development?
It promotes the differentiation of naive T cells into Tregs.
What is the difference between germline-encoded receptors and unique specificity antigen receptors?
Germline-encoded receptors are used by the innate immune system, while unique specificity antigen receptors are assembled during lymphocyte development in the adaptive immune system.
What is the significance of clonal distribution of antigen receptors in T cells?
It ensures that each T cell has a unique receptor specific to a particular antigen.
What is the role of BCR in the activation of B cells?
BCR requires two signals for activation: binding to antigen and interaction with CD40L on T cells.
What happens to B cells in the absence of specific helper T cells?
B cells become anergic, meaning they remain unresponsive.
What is BAFF and its significance for anergic B cells?
BAFF (B cell activating factor) is required for the survival of anergic B cells, which cannot compete with normal naïve B cells for it.
What is the human microbiome composed of?
The human microbiome consists of about 39 trillion bacterial cells, outnumbering human cells which number around 30 trillion.
Why does the body not attack normal gut flora?
Commensal microbes elicit little or no innate immunity and cannot invade epithelial barriers, preventing activation of the adaptive immune system.
What are AMPs and their functions?
Antimicrobial peptides (AMPs) directly kill microbes, neutralize toxins, selectively suppress harmful microbes, and maintain barriers.
What is the significance of immune privileged sites?
Immune privileged sites do not develop strong immune responses to pathogens, protecting critical tissues from damage.
What mechanisms contribute to immune privilege?
Mechanisms include lack of lymphatic drainage, blood barriers, immunosuppressive cytokines, and low MHC expression.
What is the clinical significance of immune privilege in transplantation?
Immune privileged sites, like the eye, often allow successful transplants without the need for immunosuppressive drugs.
What is the risk of microcephaly associated with Zika virus infection during pregnancy?
The estimated risk of microcephaly is 6%-13% if infection occurs during the first trimester.
How do persistent infections relate to immune privilege?
Certain viruses can persist in immune privileged sites, making them difficult to clear and increasing transmission risk.
What factors contribute to the development of autoimmune diseases?
Factors include immunologic abnormalities, genetic predisposition, infections, and environmental triggers.
What is molecular mimicry in the context of autoimmunity?
Molecular mimicry occurs when microbial antigens resemble self-antigens, potentially activating self-reactive T cells.
What is the role of autoantibodies in autoimmune diseases?
Autoantibodies mistakenly target and react with a person's own tissues, leading to autoimmune disease manifestations.
How can tissue damage lead to autoimmunity?
Damage can expose previously sequestered antigens, triggering an autoimmune response against them.
What are the two classifications of autoimmune diseases?
Autoimmune diseases can be classified as organ-specific or systemic.
What is the estimated prevalence of autoantibody-driven autoimmune diseases?
About 2.5% of the population is affected by autoantibody-driven autoimmune diseases.
What is the significance of regulatory T cells in immune privilege?
Regulatory T cells help maintain immune tolerance and prevent excessive immune responses in privileged sites.
What is the impact of low MHC expression in immune privileged sites?
Low MHC expression reduces the likelihood of immune cell infiltration and antigen presentation.
What is the role of IgA in immune defense?
IgA blocks microbes from attaching to and invading host cells and is not inflammatory.
What are the implications of immune privilege during pregnancy?
Immune privilege prevents the maternal immune system from attacking fetal cells, which express paternal antigens.
What is the connection between autoimmune diseases and gender?
Women are more likely than men to develop autoimmune diseases.
What is the significance of the blood-retinal barrier?
The blood-retinal barrier protects the eye from immune-mediated damage that could lead to blindness.
What is the relationship between infections and autoimmune responses?
Infections can activate self-reactive T cells through bystander activation or molecular mimicry.