24 Transition Metals
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
transition metal def
a d-block element which forms an ion with an incomplete d-subshell
(scandium and zinc are d-block elements, but don’t fit the rest of this definition so aren’t classified as transition elements)
electron configuration of scandium

electron configuration of zinc

d-block of periodic table
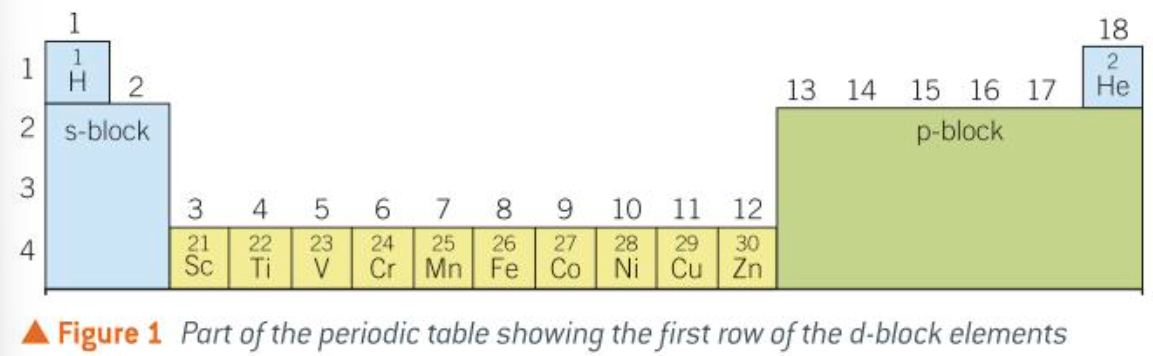
why are d-block elements called that
the 3d subshells have the highest energy and electrons are added to 3d orbitals
properties of transition metals
the incomplete d-subshell is responsible for a number of general properties of transition elements:
variable oxidation states
catalytic action
coloured compounds
formation of complexes
uses of transition metals
coinage: copper, silver, nickel, zinc
construction and the production of tools: iron
electrical cables and water pipes: copper
aerospace industry and medical applications (joint replacement and cosmetic dentistry): titanium
what are the special cases of transition metals where their subshells fill differently
chromium and copper
it’s believed that a half-filled d5 subshell and a fully filled d10 subshell give additional stability to the atoms
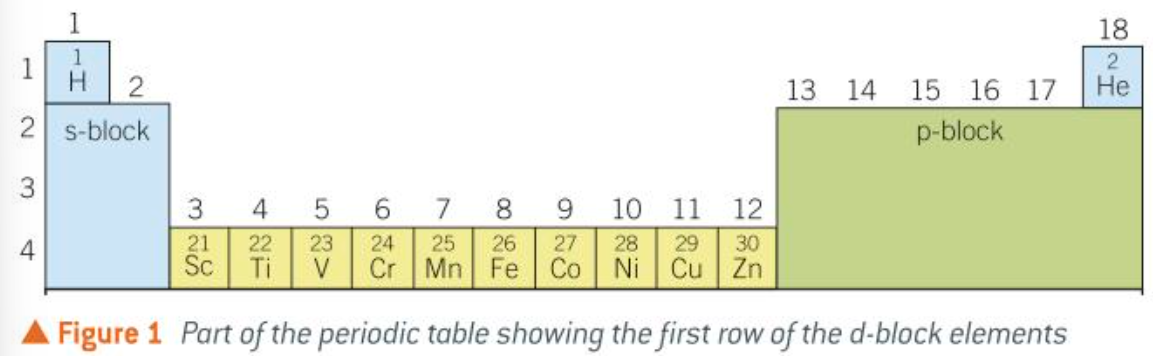
rules for electron configuration of d-block ions
when the d-block elements form positive ions from their atoms, they lose their 4s electrons before losing any of the 3d electrons.
when forming an atom, the 4s orbital fills before the 3d orbitals
when forming an ion, the 4s orbital empties before the 3d orbitals
electron configuration of Fe, Fe2+, Fe3+
how do the variable oxidation states change across the transition metals
the number of oxidation states increases across the transition elements series to manganese, then decreases.
they all form compounds with an ox no. of +2 (resulting from the loss of 2e-)
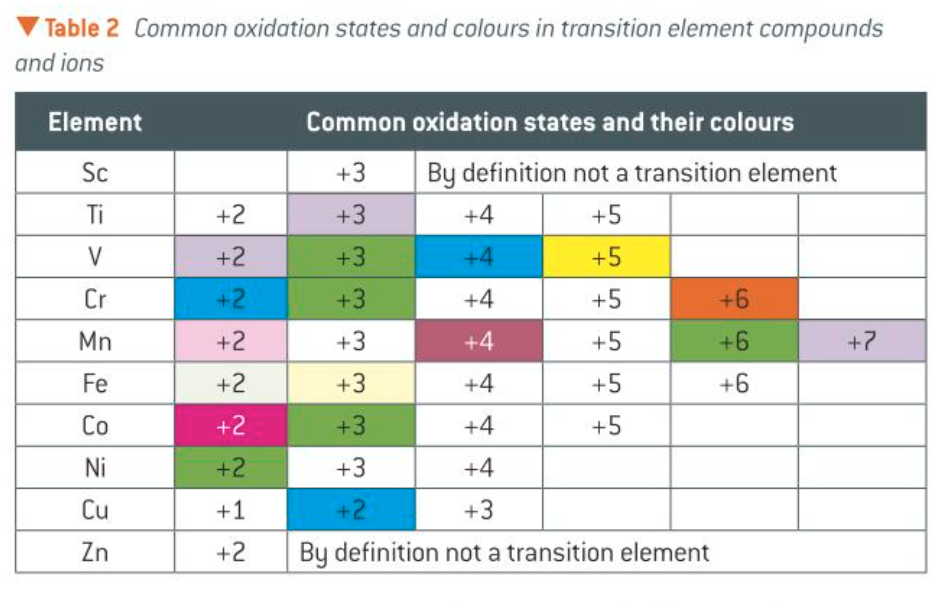
common oxidation states and their colours
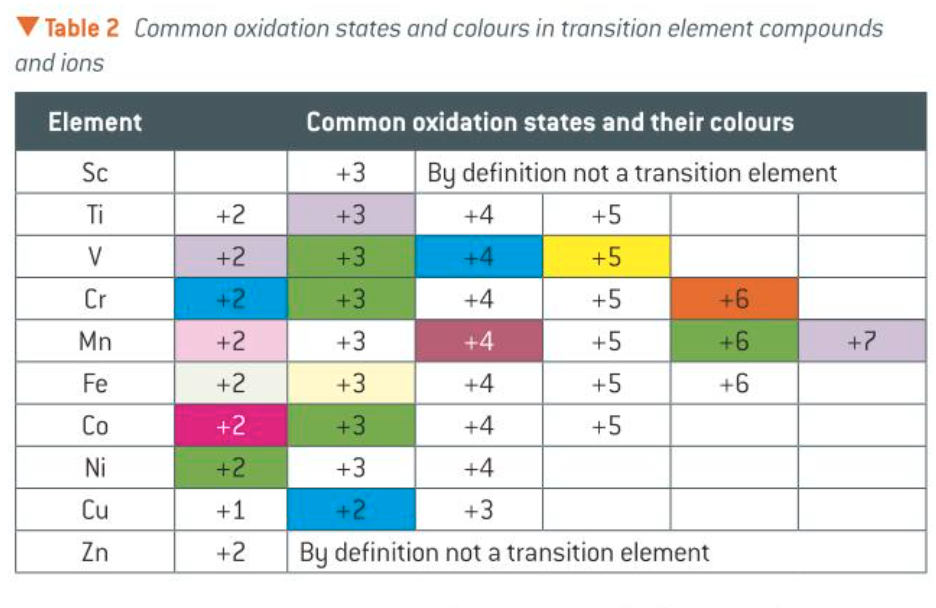
when is a species often a strong oxidising agent
when a species contains a transition element in its highest oxidation state
catalyst def
a substance that increases the rate of a chemical reaction without being used up in the process; a catalyst provides an alternative route for the reaction with lower activation energy
Contact process
the production of sulfur trioxide from the oxidation of sulfur dioxide
catalysed by vanadium (V) oxide V2O5(s)
2SO2(g) + O2(g) ⇌ 2SO3(g)
Haber process
the manufacture of ammonia from the rxn between nitrogen and hydrogen
catalysed by a finely divided iron catalyst
N2(g) + 3H2(g) ⇌ 2NH3(g)
Hydrogenation of vegetable fats in the manufacture of margarine
nickel is used as the catalyst
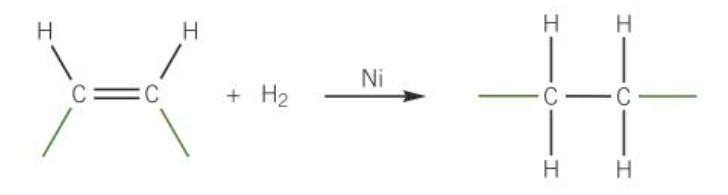
catalytic decomposition of hydrogen peroxide
forms oxygen
uses manganese (IV) oxide MnO2(s) as the catalyst
2H2O2(aq) → 2H2O(l) + O2(g)
examples of heterogenous catalysis
when the catalyst is in a different state to the reactants
catalytic decomposition of hydrogen peroxide using MnO2
hydrogenation of vegetables fats using nickel
the Contact process using V2O5 (vanadium V oxide)
the Haber process using a finely divided iron catalyst
rxn between iodide ions and peroxodisulfate ions S2O82-
catalysed by Fe2+(aq) ions
S2O82-(aq) + 2I-(aq) → 2SO42-(aq) + I2(aq)
when this rxn is carried out with a trace of starch, a blue-black colour forms showing the formation of iodine
with a small amount of Fe2+(aq) added, the blue-black solution forms much more quickly demonstrating the catalytic action of it
rxns to show how Fe2+ catalyses the rxn between iodide ions and peroxodisulfate ions S2O82-
Fe2+(aq) reacts: S2O82-(aq) + Fe2+(aq) → 2SO42-(aq) + Fe3+(aq)
Fe2+(aq) regenerated: Fe3+(aq) + 2I-(aq) → I2(aq) + Fe2+(aq)
the rxn of Zn metal with acids
catalysed by the presence of Cu2+(aq) ions
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
examples of homogenous catalysts
when the catalyst is in the same physical state of the reactants
rxn between iodide ions and peroxodisulfate ions S2O82- using Fe2+(aq)
the rxn of Zn metal with acids using Cu2+(aq)
complex
metal ion with co-ordinately bonded ligands
complex ions aren’t restricted to d-block elements (e.g. Al can form complex ions)
ligand def
a molecule or ion that donates a pair of electrons to a central metal ion to form a coordinate bond or dative covalent bond
dative covalent bond/coordinate bond def
special kind of covalent bond that’s formed when one of the bonded atoms provides both of the electrons for the shared pair
coordination number
indicates the number of coordinate bonds attached to the central metal ion
shapes of complexes
linear (Ag+ complexes)
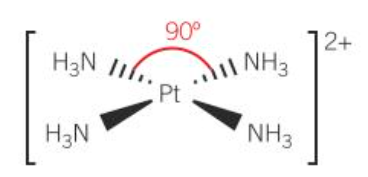
square planar (Pt2+ and Ni2+ complexes)
tetrahedral (with larger ligands e.g. Cl-, when ligands are too big for six to fit)
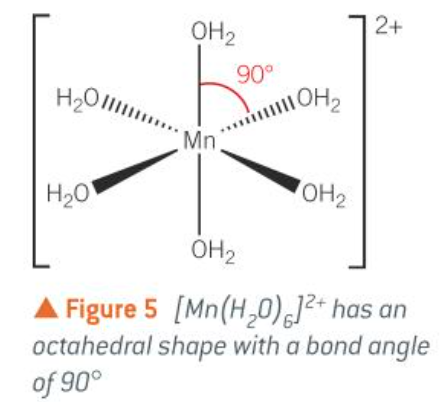
octahedral (most complexes with small ligands e.g. H2O, NH3)
examples for shapes of complexes
linear [Ag(NH3)2]+ (in Tollen’s reagent)
square planar [PtCl4]2-
tetrahedral [CuCl4]2-
octahedral [Cu(H2O)6)2+
bond angles and coordination numbers for shapes of complexes
linear: 2, 180
square planar: 4, 90
tetrahedral: 4, 109.5
octahedral: 6, 90
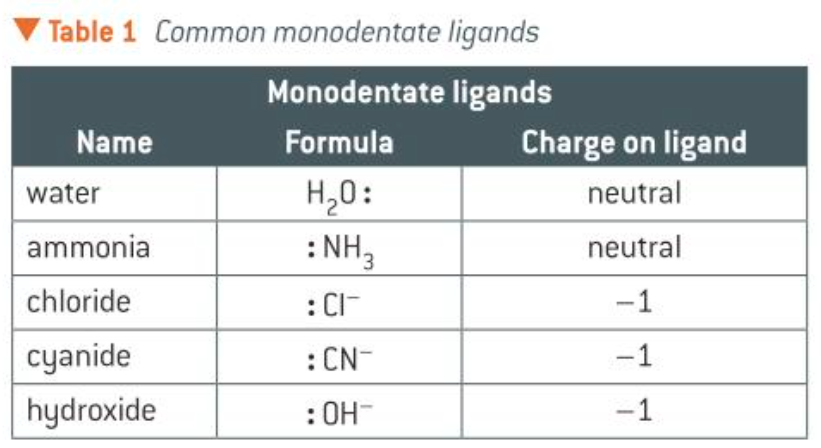
monodentate ligands
a ligand that’s able to donate 1 pair of e- to a central metal ion
common monodentate ligands
bidentate ligands
when a ligand can donate two lone pairs of e- to the central metal ion, forming 2 coordinate bonds
most common bidentate ligands
1,2-diaminoethane is shorted to en
ethandioate aka oxalate ion
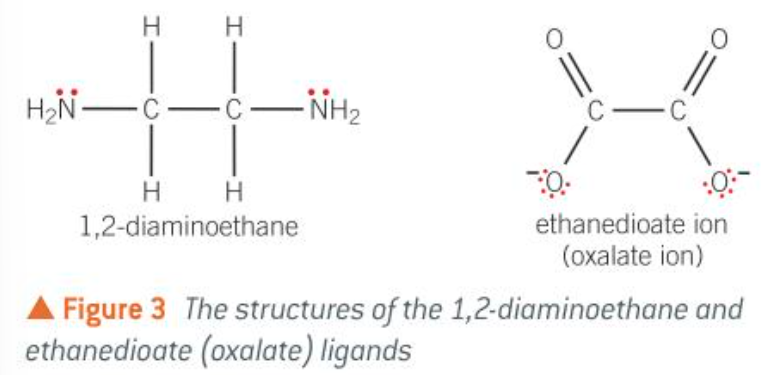
how does 1,2-diaminoethane, en, form coordinate bonds
each nitrogen atom donates a pair of e- to the central metal ion, forming a coordinate bond
how does ethanedioate form coordinate bonds
each negatively charged oxygen atom donates a pair of electrons to the central metal ion
six coordinate complex shape
four coordinate complexes: tetrahedral shape
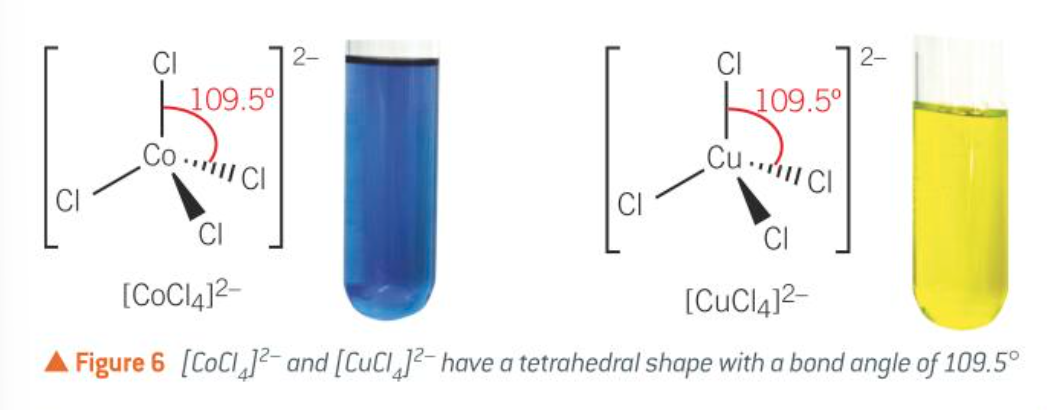
four coordinate complexes: square planar complexes
stereoisomer def
compounds with the same structural formula but with a different arrangement of the atoms in space
optical isomers
stereoisomers that are non-superimposable mirror images of each other
what does the type of stereoisomerism of a complex ion depend on
the number and type of ligands that are attached to the central metal ion
the shape of the complex
some 4-coordinate and 6-coordinate complex ions containing two different monodentate ligands show cis-trans isomerism
some 6-coordinate complex ions containing monodentate and bidentate ligands can show both cis-trans and optical isomerism
cis-trans isomerism in complex ions
the shape of the complex holds groups in different orientations about the central metal ions
cis-trans isomerism occurs in some square planar and octahedral complex ions
cis-trans isomerism in square planar
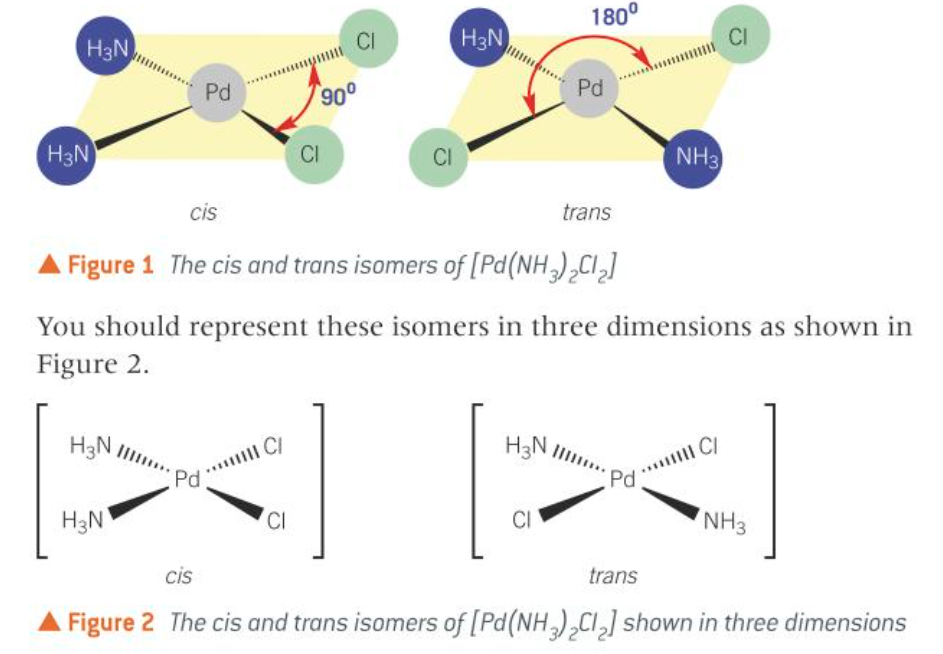
cis-trans isomerism in octahedral complexes with monodentate ligands
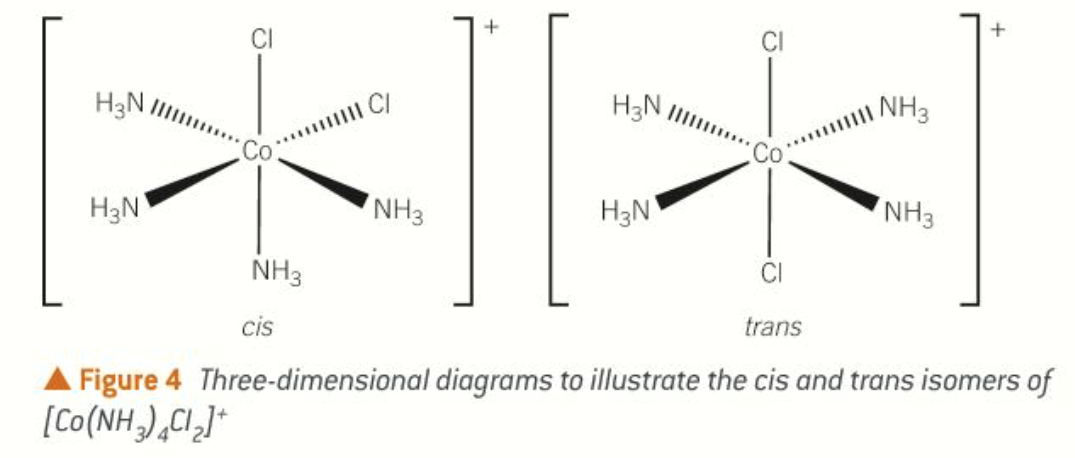
cis-trans isomerism in octahedral complexes with bidentate ligands
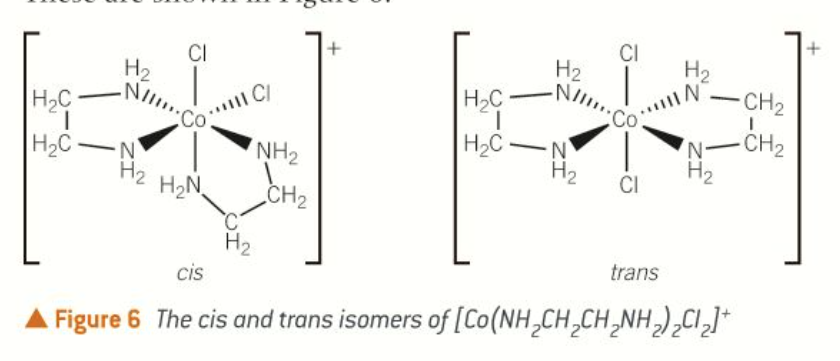
optical isomerism in octahedral complexes
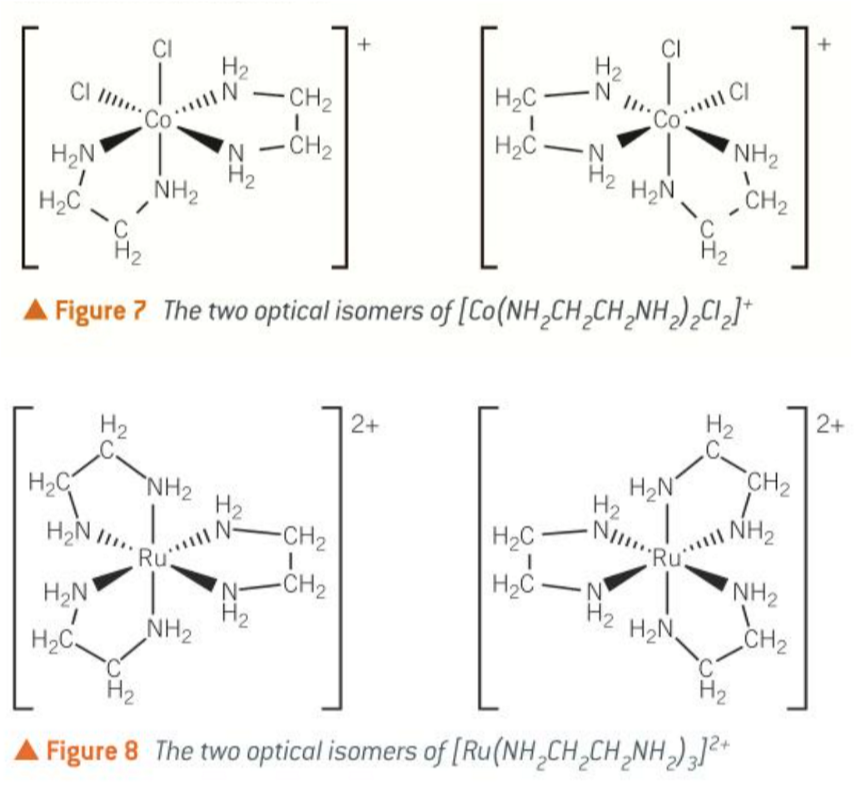
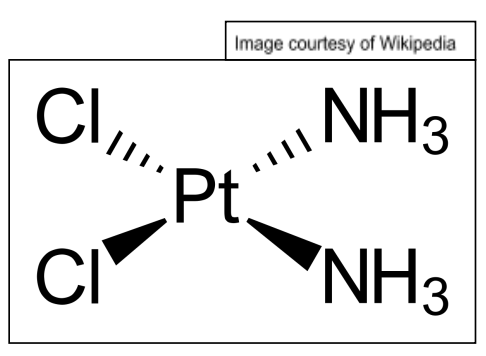
cis-platin
cisplatin is the cis isomer of a square planar complex of platinum
it has both chlorine atoms on the same side
it’s commonly used as a cancer therapy drug
drugs like cisplatin target components of cells that are chiral, so only one isomer of the drug will be the correct orientation to ‘fit’ the cells.
cisplatin can cause serious side effects such as hair loss, meaning it has to be administered in small amounts to try and reduce these effects while still targeting the cancerous cells
ligand substitution
when one ligand in a complex ion is replaced by another ligand
what happens when CuSO4 is dissolved in water
the pale blue complex ion [Cu(H2O)6]2+ is formed in (aq)
what happens when XS NH3(aq) is added (dropwise) to a solution containing [Cu(H2O)6]2+
the pale blue solution colour changes to form a dark blue solution
when dropwise:
a pale blue ppt of Cu(OH)2 forming in the first stage of the rxn
the Cu(OH)2 ppt dissolving in XS NH3 to form a dark blue solution
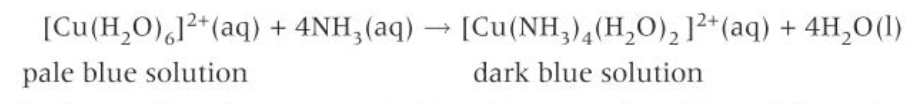
what happens when an XS of conc HCl is added to a solution containing [Cu(H2O)6]2+
pale blue solution changes to form a yellow solution
if water is added to the yellow solution, a blue solution will be formed, although more dilute and paler in colour than the original blue solution
if you take care making observations, you will see an intermediate green solution (the result of the two solutions mixing)
what happens when KCr(SO4)2 • 12(H2O) (aka chrome alum) is dissolved in water
the complex ion [Cr(H2O)6]3+ (a pale purple solution) is formed
![<p>the complex ion [Cr(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup> (a pale purple solution) is formed</p>](https://knowt-user-attachments.s3.amazonaws.com/87fc39d2-86cb-4c1e-b52a-4a9d69bb4a3d.png)
what happens when Cr2(SO4)3 is dissolved in water
a green solution containing Cr3+ is formed - [Cr(H2O)5SO4)+ where one of the H2O ligands has been replaced by a the SO42-
what happens when you add XS NH3(aq) dropwise to [Cr(H2O)6]3+
initially a grey-green ppt of Cr(OH)3 is formed
the Cr(OH)3 ppt dissolves in XS NH3 to form the complex ion [Cr(NH3)6]3+
![<ol><li><p>initially a grey-green ppt of Cr(OH)3 is formed</p></li><li><p>the Cr(OH)3 ppt dissolves in XS NH3 to form the complex ion [Cr(NH<sub>3</sub>)<sub>6</sub>]<sup>3+</sup></p></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/f296c92c-ecef-4a40-bf27-6163939cda6b.png)
equation for what happens when you add XS NH3(aq) dropwise to [Cr(H2O)6]3+
[Cr(H2O)6]3+(aq) (violet) + 6NH3(aq) → [Cr(NH3)6]3+(aq) (purple) + 6H2O(l)
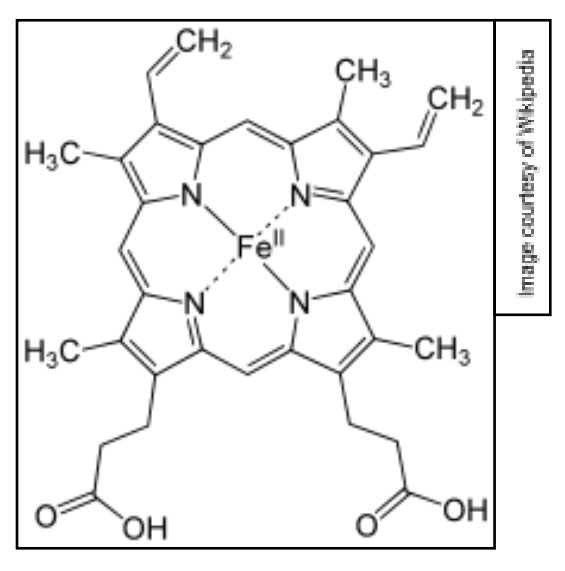
haemoglobin
contains four protein chains held together by weak IMF
each protein has a haem molecule within its structure
the central metal ion in a haem group is Fe2+ which can bind to oxygen gas O2
haem
a component of haemoglobin is another common example involving multidentate ligands
it consists of a central Fe2+ ion and a tetradentate porphyrin ring
the central Fe2+ ion can also form coordinate bonds with one or two additional axial ligands
this gives haemoglobin an overall octahedral structure
the shape and structure allow it to transport oxygen around the body
a ligand substitution rxn occurs when the oxygen usually bound to haem is replaced with carbon monoxide CO.
the CO binds more strongly than oxygen so can’t be removed. therefore CO is toxic to humans as it prevents oxygen being transported around the body
colour of MnO4- and Mn2+
purple to colourless

redox rxn between Fe2+(aq) and MnO4- (manganate VII ions)
Fe2+ is oxidised to Fe3+
MnO4- is reduced to Mn2+
the solution containing MnO4- ions is purple and is decolourised by Fe2+(aq) to form a colourless solution containing Mn2+(aq)
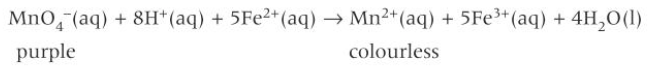
reduction of Fe3+ to Fe2+ by I-(aq)
orange-brown Fe3+(aq) are reduced to pale green Fe2+(aq)
but this is obscured by the oxidation of the I- to form I2(aq) which has a brown colour
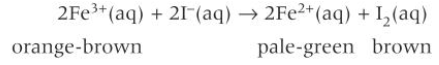
reactions for the reactions of Cr2O72- and Cr3+ (with and without Zn)
Cr2O72-(aq) + 14H+(aq) +3Zn(s) → 2Cr3+(aq) + 7H2O(l) + 3Zn2+(aq)
with XS Zn:
Zn(s) + 2Cr3+(aq) → Zn2+(aq) + 2Cr2+(aq)
equation for oxidation of Cr3+ to CrO42-
3H2O2 + 2Cr3+ + 10OH- → 2CrO42- + 8H2O
reduction of Cu2+ to Cu+
when Cu2+(aq) reacts with XS I-(aq), forms a white ppt but I2 is a brown solution so ppt masked
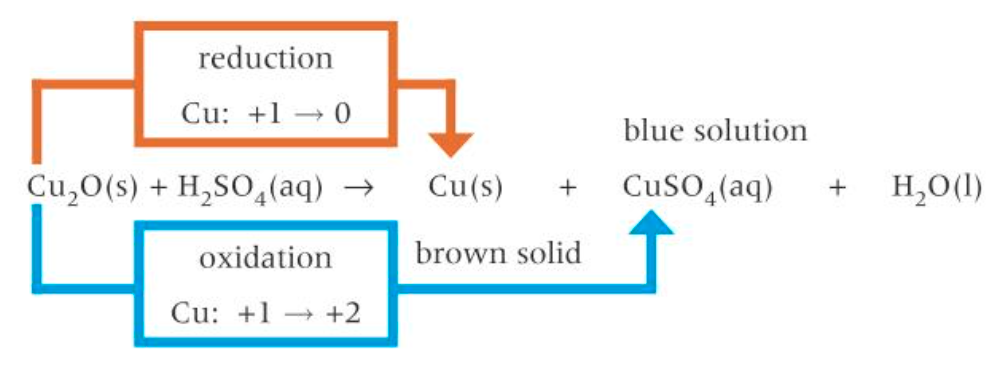
disproportionation of Cu+ ions with hot dilute sulfuric acid
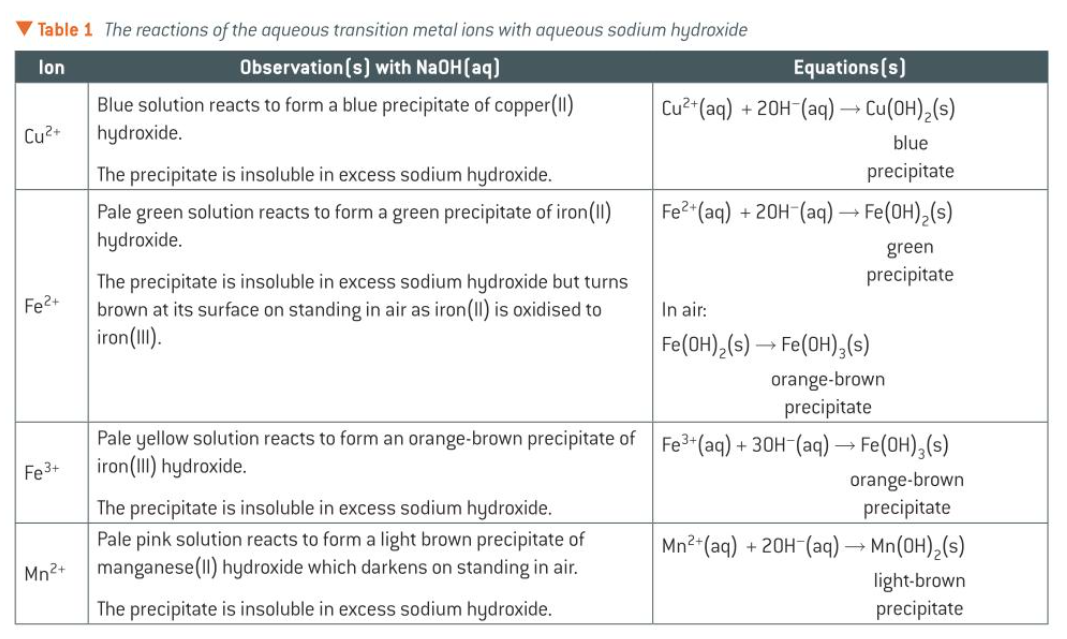
identifying cations
transition metal ions:
NaOH(aq) produces ppts with aqueous TM ions
these rxns can be used for identifying TM ions in an unknown solution
need to know the observations for these rxns
NH4+:
when heated with OH- ions, NH4+ reacts to produce NH3
NH4+(aq) + OH-(aq) → NH3(g) + H2O(l)
heat gently
test w damp red pH indicator paper will turn blue
identifying anions