CHAP 12: Thermodynamics
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
In a cathode ray tube, electrons flow from the negatively charged cathode to the positively charged anode. Cathode ray tubes may be small and compact, but they typically must be plugged in to function. The flow of electrons within this tube is:
nonspontaneous (requires input energy)
Dispersal of matter and energy can be directly related to spontaneity if the:
Select the correct answer below:
change in internal energy is positive
change in internal energy is negative
change in internal energy is zero
there are no conditions for this correlation
change in internal energy is zero
(When a process has no change in internal energy, an increase in the dispersal of matter and energy will always be spontaneous.)
Which phase change involves an increase in the dispersal of matter?
Select the correct answer below:
deposition
condensation
freezing
sublimation
Sublimation is the phase change from solid directly to gas.
This results in a large increase in the dispersal of matter because gas particles are much more spread out and move more freely than solid particles.
Identify the option below that is a spontaneous process:
The conversion of rust to iron
Water flowing downhill
Water flowing uphill
Boiling water on a stovetop
Water flowing downhill; Spontaneous processes occur naturally under certain conditions. They do not need to be driven by the continual input of energy from an external source.
Which process is nonspontaneous?
water freezing at room temperature and atmospheric pressure
water freezing at -30C
water boiling at 115C
all of the above
water freezing at room temperature and atmospheric pressure
Under ambient conditions, diamonds are said to be:
thermodynamically stable and kinetically stable
thermodynamically unstable and kinetically unstable
thermodynamically stable but kinetically unstable
thermodynamically unstable but kinetically stable
thermodynamically unstable but kinetically stable;
Thermodynamically unstable: Under standard (ambient) conditions, graphite is the more stable form of carbon, not diamond. So diamonds are not the most stable form from a thermodynamic standpoint — they "want" to convert to graphite.
Kinetically stable: However, the conversion from diamond to graphite is extremely slow because it has a very high activation energy. That means the reaction basically doesn’t happen on its own — it's kinetically stable.
Two flasks are connected by a closed valve. One contains gas particles and the other contains a vacuum. If the valve is opened such that the particles move until they fill both flasks:
the internal energy of the system has increased
the thermal energy of the system has decreased
the thermal energy of the system remains the same
impossible to calculate
the thermal energy of the system remains the same;
This is a free expansion of a gas into a vacuum (also called Joule expansion), and it has some key characteristics:
No work is done (because the gas expands into a vacuum — there's no opposing pressure)
No heat is exchanged (assuming the system is isolated)
So by the first law of thermodynamics:
ΔU=q+w=0+0=0
→ Internal energy stays the same
Entropy is a state function because:
it depends only on the initial and final states of a system.
Who first developed the concept of entropy?
Clausius
A microstate is…
A microstate is a possible configuration or arrangement of matter and energy within a system.
Which phase change does NOT have a positive value for the change in entropy?
liquid to solid;
Freezing involves an decrease in entropy because particles are becoming more ordered. Since entropy is a measure of disorder, entropy will decrease when a liquid freezes into a solid. This will result in a negative value for the change in entropy.
Of the following, which is an example of a decrease in entropy?
A change from the liquid state to the gaseous state
A change from the liquid state to the solid state
A change from the solid state to the gaseous state
A change from the liquid state to the solid state
For any substance:
Solid < Liquid < Gas
This means that a phase change from a liquid to a solid (crystallization) decreases entropy.
A system has 4 particles distributed among
2 boxes. Which of the following is the most probable configuration?
a configuration with 2 particles in each box;
The configuration with 2 particles in each box is the most probable distribution, since the probability that a system will exist with its components in a given distribution is proportional to the number of microstates within the distribution. In a system of 4 particles, a distribution with 2 particles in each box has the greatest number of microstates.
In reality, all natural processes are:
irreversible
A grouping of microstates with equivalent particle arrangements is called a(n):
distribution;
A distribution is a collection of all the microstates with equivalent particle arrangements, if we consider all particles to be identical.
Which of the following is the definition of a "distribution" as it relates to the microstates of a system?
a group of microstates with equivalent particle arrangements
A distribution is a group of microstates with equivalent particle arrangements, assuming that all particles are identical.
Choose the options below that are true for the entropy of a substance:
Heavier atoms possess greater entropy at a given temperature than lighter atoms.
✔ True — Heavier atoms have more available vibrational and rotational modes, increasing their entropy.
The entropy of a substance is influenced by the structure of the particles that comprise the substance.
✔ True — Molecular complexity (e.g., branching, number of atoms, flexibility) increases the number of ways energy can be distributed → higher entropy.
Which of the following is true if the temperature difference between a particular system and its surroundings is infinitesimally small? Select all that apply.
Select all that apply:
Heat flow is thermodynamically reversible.
No heat can flow between the system and the surroundings.
The entropy of the universe does not change.
The entropy of the universe must decrease.
Heat flow is thermodynamically reversible.
✔ True — If the temperature difference is infinitesimally small, the system is in thermal equilibrium with its surroundings, and heat can flow reversibly. This is a hallmark of a reversible process.
The entropy of the universe does not change.
✔ True — In a reversible process, the total entropy change of the universe is zero. The entropy gained by the system is exactly balanced by the entropy lost by the surroundings (or vice versa).
Which of the following summarizes the second law of thermodynamics?
The second law of thermodynamics states that the entropy of the universe increases for a spontaneous process.
The entropy of a substance above absolute zero will always be:
Select the correct answer below:
negative
positive
zero
depends on the temperature
POSITVE;
Entropy (S) is a measure of disorder or the number of microstates available to a system.
According to the third law of thermodynamics, the entropy of a perfect crystal at absolute zero is zero.
As soon as the temperature rises above absolute zero, particles begin to move, and entropy increases — even if just slightly.
So:
At 0 K → entropy = 0 (perfectly ordered crystal)
Above 0 K → entropy becomes positive
Write an equation or an inequality for the entropy of an unidentified crystalline solid at room temperature.
S > 0
According to the third law of thermodynamics:
At absolute zero (0 K), a perfect crystal has zero entropy →
𝑆
=
0
S=0
At any temperature above 0 K (like room temperature), the entropy becomes positive due to increased microstates
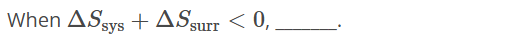
Change in Entropy of the Universe is negative
A process will definitely be spontaneous if:
Select the correct answer below:
the entropy of the system increases in the process
the entropy of the system decreases in the process
the entropy of the universe increases in the process
the entropy of the universe decreases in the process
the entropy of the universe increases in the process
The standard entropy of a substance refers to its entropy at:
25 degrees Celsius & 1 Bar
Which of the following is the change in entropy for a reaction calculated using standard entropy values?
Standard entropy change is defined as the change in entropy for a reaction calculated using the standard entropies.