1.1 Elements: The "building blocks" of all materials
0.0(0)
Card Sorting
1/10
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
1
New cards
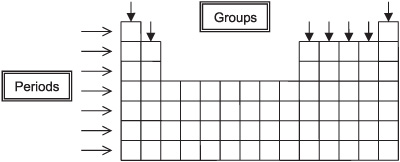
A vertical column in the periodic table that contains elements with similar chemical properties
Alkali Metals (Group 1)
Noble Gases (Group 18)
Alkaline Earth Metals (Group 2)
Halogens (Group 17)
What is the Group of a Periodic Table? What are some examples of groups?
2
New cards
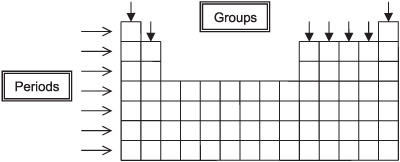
horizontal row in the periodic table where elements have the same number of electron shells
Period
3
New cards
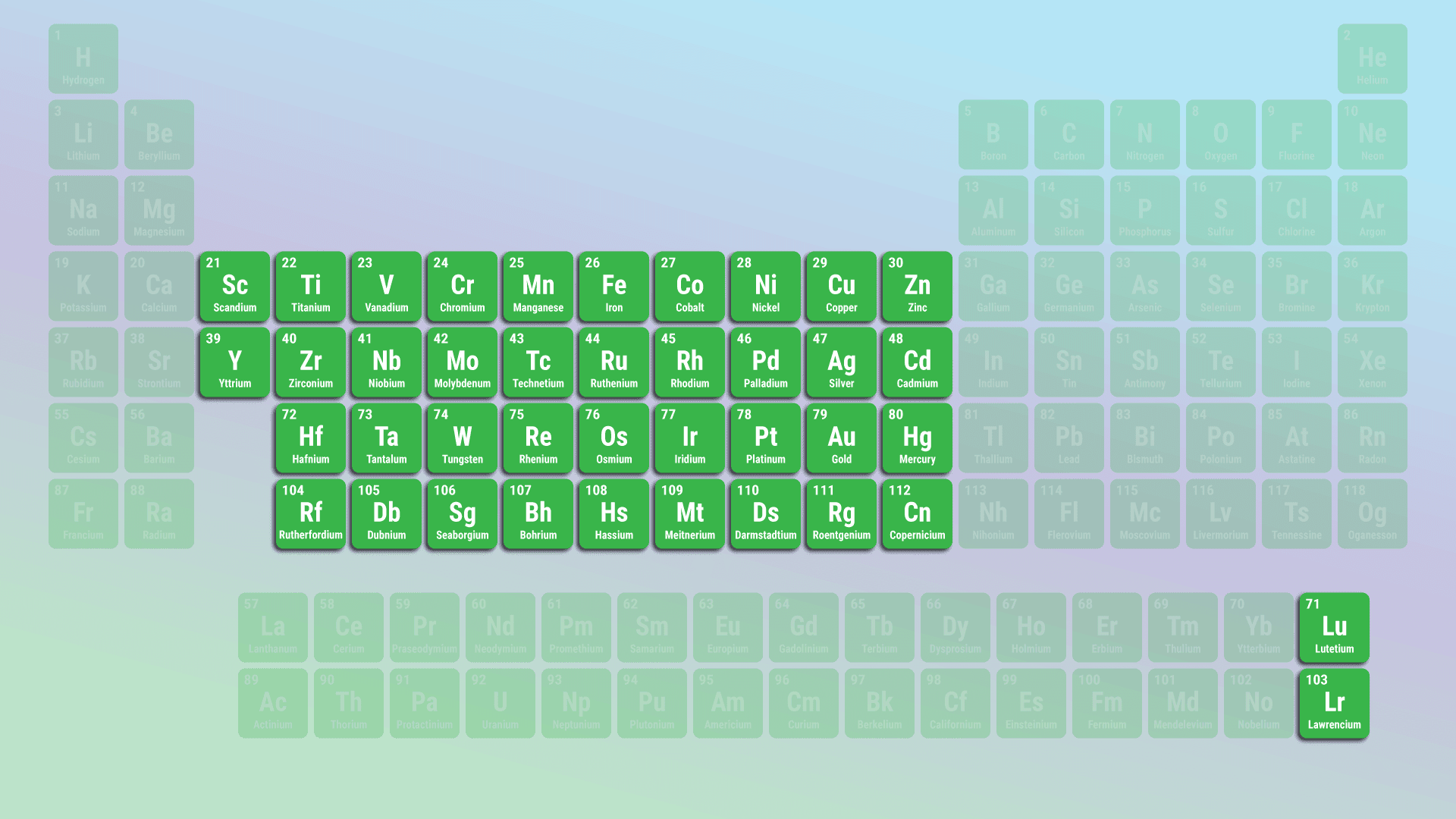
Elements found in the centre of the periodic table (groups 3–12) that are typically hard, dense, and good conductors
Transition Metals
4
New cards
highly reactive metals in Group 1 such as sodium (Na) and potassium (K)
Alkali Metals
5
New cards
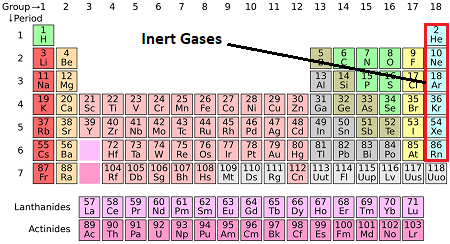
Inert, nonreactive gases in Group 18
ex. helium and neon
Noble Gases + examples
6
New cards
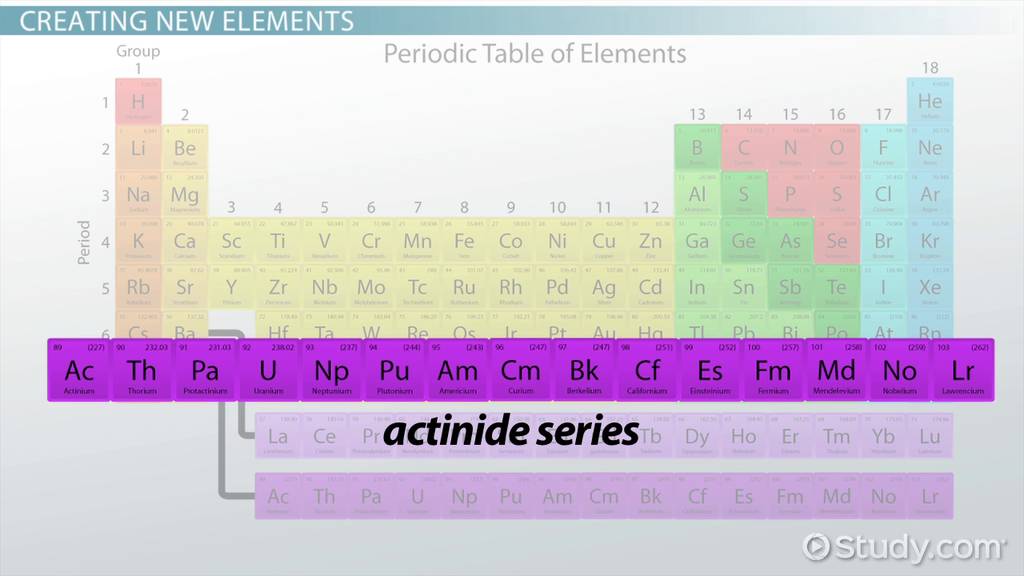
A series of radioactive elements from actinium to lawrencium, found in the f-block of the periodic table
Actinoids
7
New cards
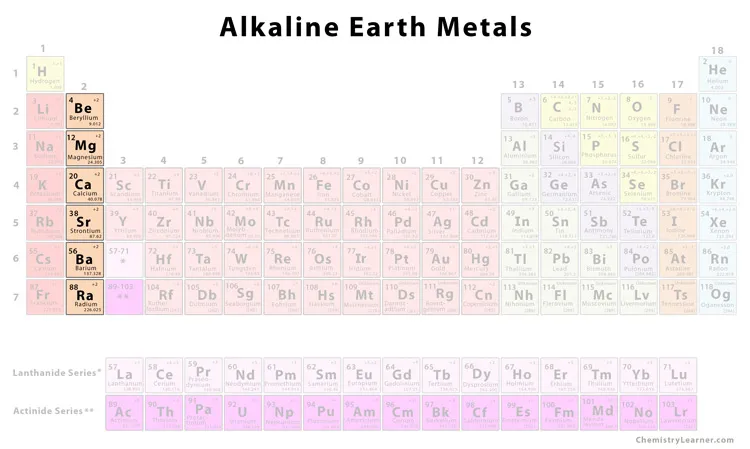
reactive metals in Group 2
ex. calcium and magnesium
Alkaline-Earth Metals + examples
8
New cards
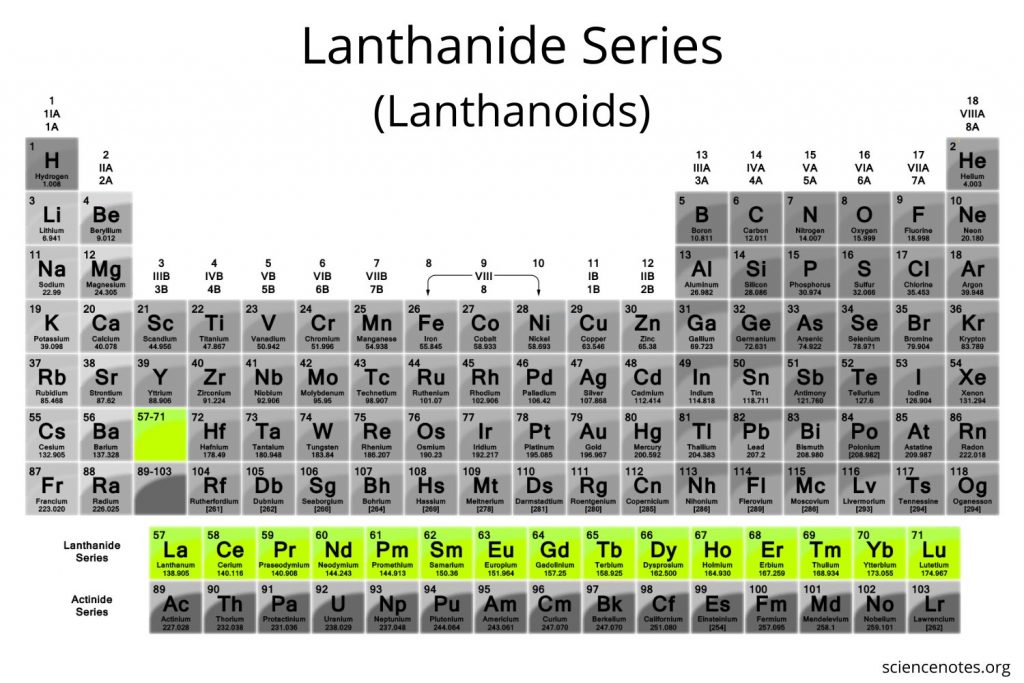
a series of elements from lanthanum to lutetium, known for forming bright-coloured compounds
Lanthanoids
9
New cards
very reactive nonmetals in Group 17
such as fluorine and chlorine
Halogens + examples
10
New cards
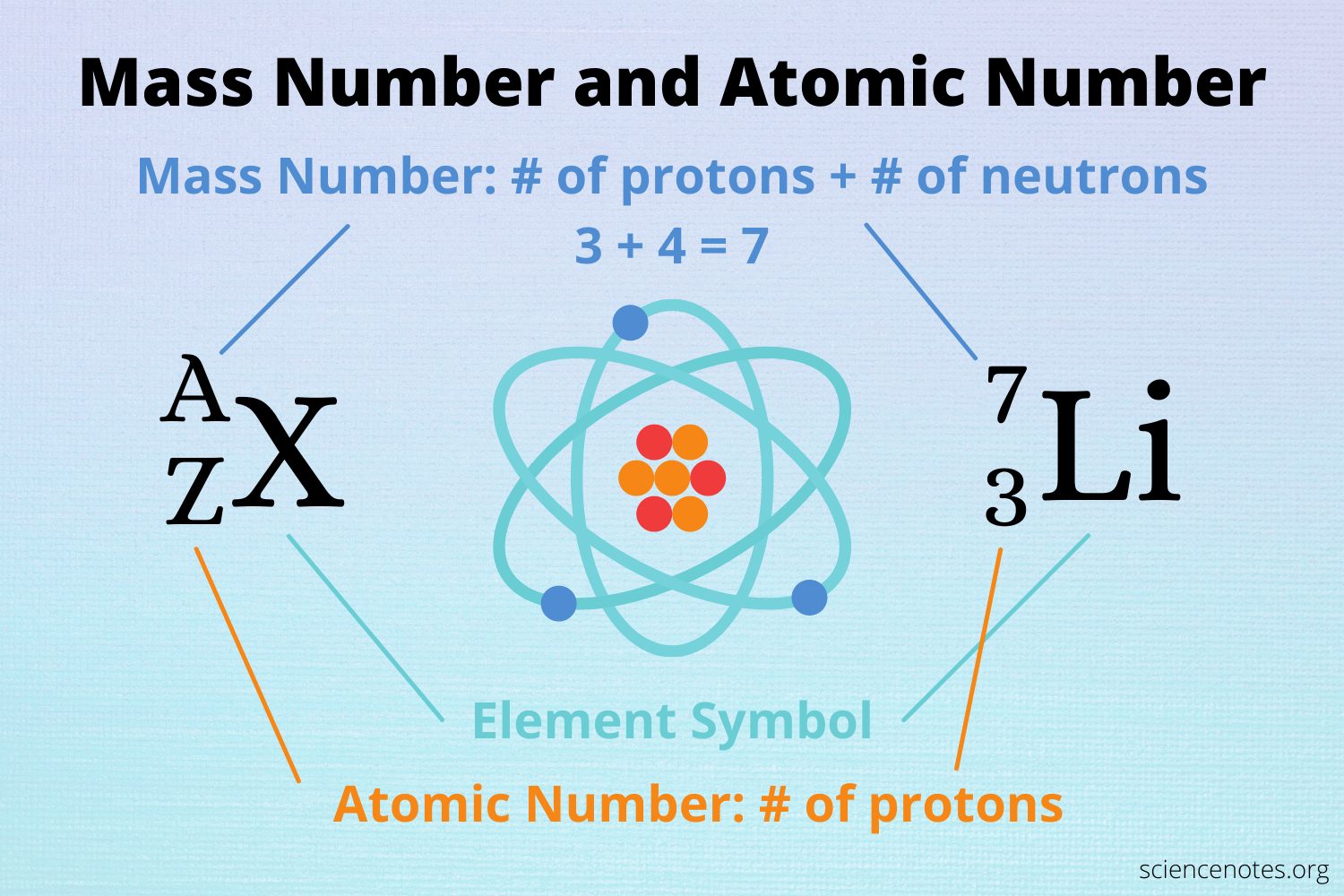
the number of protons in the nucleus of an atom, which determines the element
Atomic Number
11
New cards
The weighted average mass of an element’s isotopes, measured in atomic mass units (amu)