Core practical 16 - Synthesise aspirin from 2-hydroxybenzoic acid
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
Which functional group of the 2-hydroxybenzoic acid reacts with the ethanoic anhydride?
The hydroxyl group.
Draw the structural formulae for the reactants and product involved in the formation of aspirin from 2-hydroxybenzoic acid.
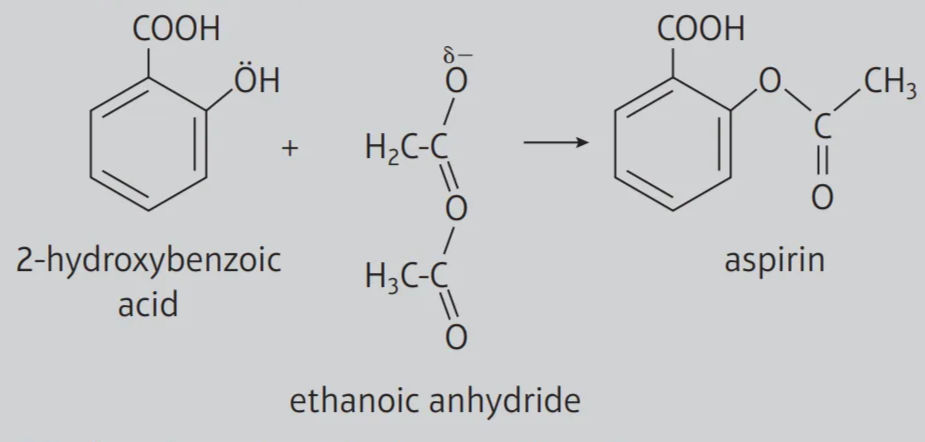
Calculate the relative molecular masses of 2-hydroxybenzoic acid and aspirin.
2-hydroxybenzoic acid = 138; aspirin = 180
Calculate the theoretical yield.
2.6g
Calculate the percentage yield.
This will vary if you use your own results: the yield / 2.6 × 100; using sample data gives 81%.
Why might the apparent yield be higher?
Because of impurities in the sample; the crystals may not be dry
What would you expect to be the main impurity in your sample?
Unreacted 2-hydroxybenzoic acid.
The actual melting temperature of aspirin is 136 °C. Is this similar to the value you recorded? Why do you think there might have been a difference?
You should record a melting temperature range rather than a single temperature. This is because impurities in the sample cause the solid to melt over a temperature range, rather than sharply at one temperature. The narrower the range and the closer your value to 136°C, the purer the sample.