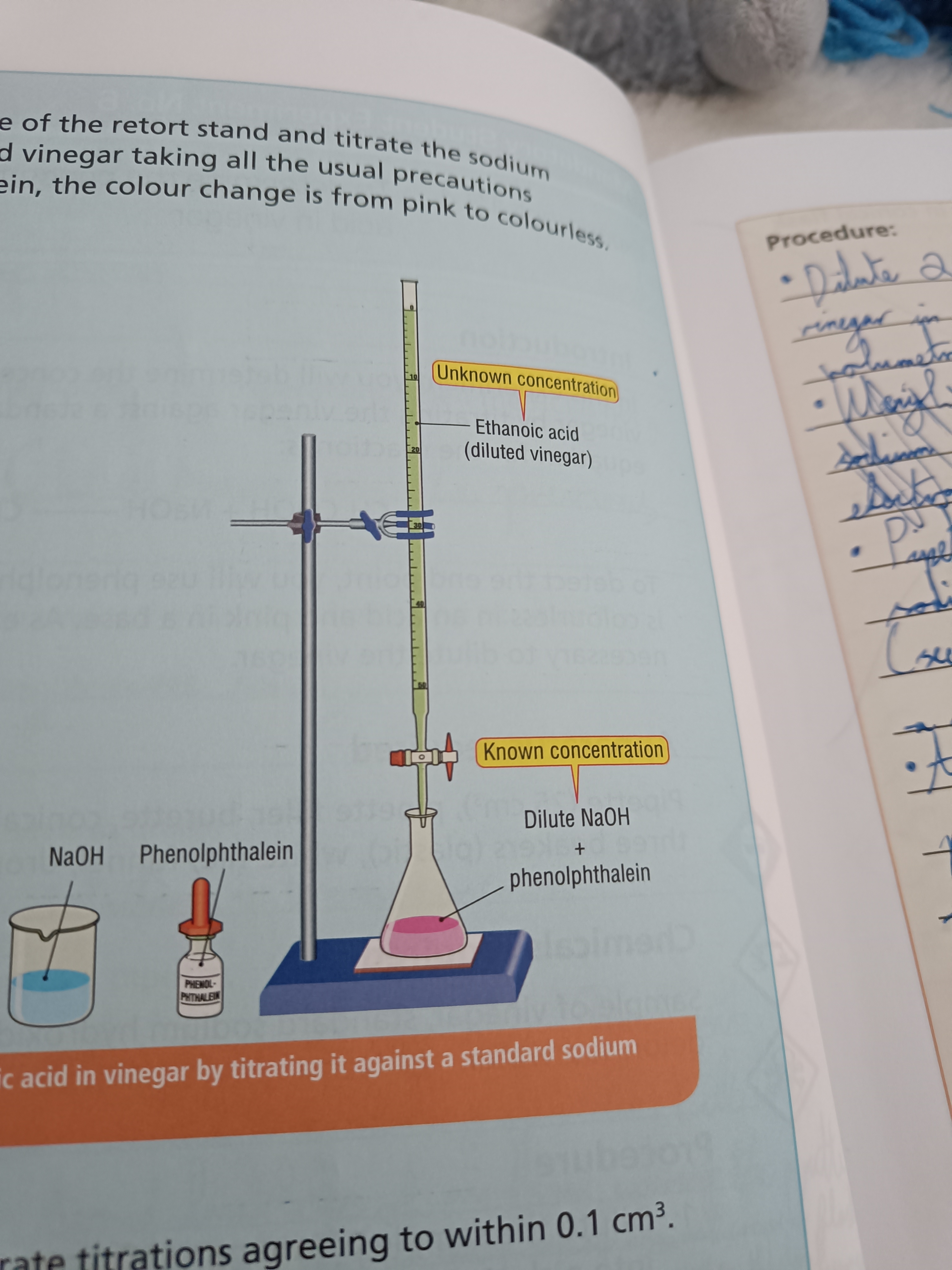
To determine the concentration of ethanoic acid in vinegar
0.0(0)
Card Sorting
1/10
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
1
New cards
Step 1
Dilute vinegar in a volumetric flask
2
New cards
Step 2
Pipette sodium hydroxide solution into a conical flask
3
New cards
Step 3
Add phenolphthalein to the conical flask
4
New cards
Step 4
Rinse burette with vinegar solution and fill it to the 0 mark
5
New cards
Step 5
Add acid as conical flask is swirled
6
New cards
Step 6
Continue until sodium hydroxide turns from pink to colourless and repeat twice
7
New cards
Why is vinegar diluted?
Ensures only a small amount of base is needed
8
New cards
Colour change of this experiment
CPink to colourless
9
New cards
What salt is formed in this experiment?
Sodium ethanoate
10
New cards
Safety percautions (2)
Wear safety goggles. Lab coat
11
New cards
Draw a diagram of this experiment