(1) Biological Organization, Water, Molecular Interactions, and Acid-Base Chemistry
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
Cellular Dimensions
Size of these cells are going to be dependent on how much O2 needs to be transported from the external to internal part of the cell
ALL cell need O2 for energy
The size + shape of the cell is going to be dependent on how much O2 you need to get to the diff. compartments within the cell
Upper limit of cell size is likely set by the rate of transport + need to deliver O2 to all parts of the cell
Structural Features of Animal Cells
Each organelle enclosed within a lipid bilayer
Cell has multiple biomolecules that must get from outside of the cell into the cell
Cell is built on multiple biomolecules
Cells Build Supramolecular Structures
Monomeric Units → Macromolecules → Supramolecular Complexes
Monomeric Units: most basic unit
Macromolecules: polymers utilize non-covalent bonds to accurately fold to form macromolecules
Subunits held together by covalent bonds
Supramolecules + Macromolecules are held together by large number of weaker interactions
Noncovalent interactions (hydrogen bonds)
Ionic interactions, van der Waals interactions, hydrophobic effect
Biomolecules
Carbon = Major Component
Chemistry of living things are organized around the properties and interactions of carbon
These bonds will determine the characteristic of the molecule that is formed
Geometry of Carbon Bonding
The lightest elements form the strongest bonds
Carbon can form covalent bonds w/ up to 4 other carbons to build linear and branched chain
Tetrahedral
Single C-C bond = flexible + can rotate b/t each molecule
C=C is very fixed
Planar = can both have cis + trans config.
Carbon can form single bonds w/ hydrogen, sulfur, and phosphorus
Can form single and double bonds w/ oxygen and nitrogen
When carbon interacts w/ something that is more electronegative, then there’s going to be a dipole
Functional Groups of Biomolecules
Biomolecules = FGs attached to carbon
FGs important in determining the chemistry / personality of protein
Acetyl-coenzyme A → important for respiration + lipid oxidation + lipid synthesis
Molecular Composition of Human Cells
# Molecules = use Avogadro’s number
Molecule % = highest % of the cell is water
Shape of the cell will depend on the requirement of diffusion of O2 as well as these biomolecules
Biomolecules restricted to stay within the cell by being phosphorylated
Limits on Cell Size
Lower Limit = size of required biomolecules
Upper Size = rate of solute molecular diffusion in an aqueous environment
Four Major Classes of Biological Macromolecules
Nucleic Acids, e.g. DNA
Store & transmit info.
Proteins, e.g. hemoglobin
Structure & catalysis
Lipids, e.g. phosphatidylcholine
Membranes & energy storage
Convert carbon-based molecules to energy
Polysaccharides, e.g. bacterial surface
Energy storage, structure, surface recognition
Carbohydrates form cellulose in plants
Building Blocks of Biochemistry
All cellular activity are the foundation of life
These building blocks are used to build polymers
These polymers fold into their appropriate conformation, and when they fold, they’re going to use non-covalent bonds to build the supramolecular structures
Parent Sugar = alpha-D-Glucose
Bioenergetics, Thermodynamics & Metabolism
Gibbs Free Energy, G: amount of energy in a rxn at constant temp. and pressure
Required to do work
Enthalpy, H: heat of a rxn reflecting the # and kind of chemical bonds in reactants and products
Heat energy ABSORBED = endothermic
Heart RELEASED = exothermic
Usually spontaneous
Entropy, S: randomness or disorder in a system
Positive = increasing randomness
Spontaneous rxns occur when ΔG is negative
Or when ΔH is highly negative, exothermic
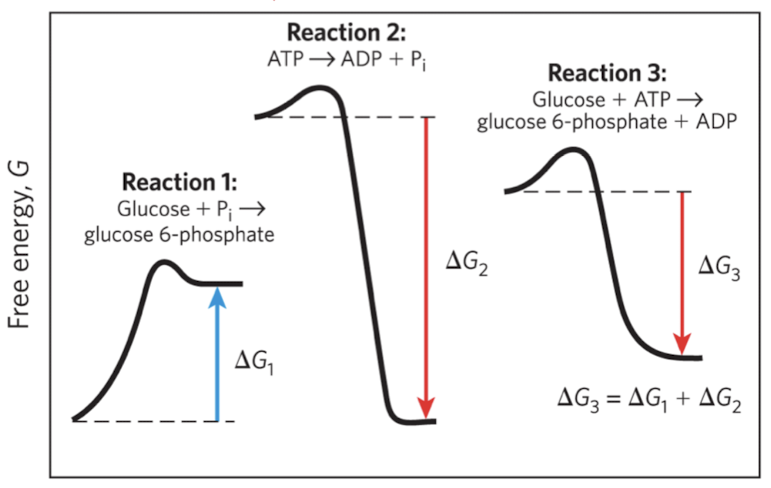
Reaction 1: if the product is still above the original energy of the starting product, ΔG is positive
Reaction 2: if the product is below the energy of starting product, ΔG is negative
Reaction 3: coupled rxn → ΔG is positive and it need energy
Coupling Reactions
Energy-requiring (endergonic) rxns are often coupled to rxns that release free energy (exergonic)
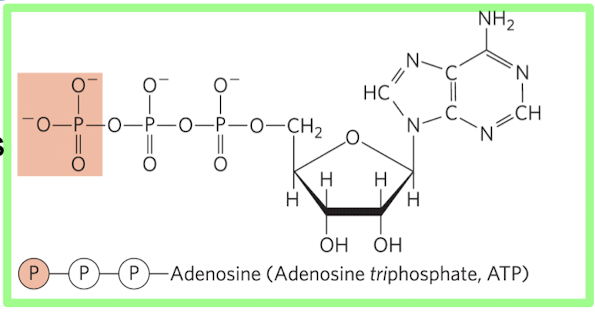
Breakage of phospho-anhydride bonds in ATP = Highly Exergonic
Structure of ATP
ΔG° = -RTlnKeq
Keq and ΔG° are measures of a reaction’s tendency to proceed Spontaneously
Genetic Principles
The structure of DNA allows its replication and repair w/ near-perfect fidelity
Dexyribonucleotides (DNA) = monomeric subunit that make up the DNA polymer
The central dogma of pretty much every cell is the nucleic acid
Each DNA NATIVE conformation = precise 3-D structure of protein
Crucial to protein function
Ribonucleotide in one strand pairs specifically w/ a complementary DNA in opposite strand
Strands are held together by hydrogen bonds
Changes in Hereditary allow Diversity
Native Conformation = precise 3D structure of a protein
Crucial to protein function
Mutation = changes in the nucleotide sequence of DNA
Changes the instructions for a cellular component
Can be beneficial
Wild Type = unmutated cells
Original state of your protein
Water
Most intermediates of metabolites, nucleic acids, and proteins are soluble in water
Lipid bilayers form spontaneously in water + stabilized by their interaction w/ it
Ionization Behavior of Water → Weak acids and bases dissolved in water can be represented by one or more equilibrium constants
Buffer = aqueous solution of a weak acid + its salt
Resists changes in pH
Hydrogen Bonds, Ionic Interactions, and Hydrophobic Effect = individually weak
Combined effect influence the 3D shape and stability of biological molecules and structures
Structure of Water & Hydrogen Bonding
Dipolar Nature of Water
Hydrogen atoms = Localized partial positive charged
Oxygen atom = Partial negative charge
Tetrahedral Arrangement
Electron pairs and hydrogen atoms around oxygen atoms
Hydrogen Bonds
Longer and weaker than covalent O-H bond
Hydrogen Bonding in Water
4 hydrogen bonds possible per water molecule
Entropy Effect: important for clathrate-like structure
Lifetime of Hydrogen Bond: liquid water can be described as a flickering structure
Water typically form these cage-like structures, especially around non-hydrophobic molecules
Thermodynamics of Water
Melting or Evaporation
Require heat from environment
Entropy of the aqueous system increase (+ΔH)
Room Temp. = Melting and evaporation occur spontaneously
ΔG must be negative
Because ΔH is positive
Increase in ΔS drives these changes
Hydrogen Bonds in Biochemistry
Hydrogen bond donors
N-H
O-H
NOT C-H
Will NOT have hydrogen bonds b/t carbon and carbon → carbon share abt. the same electronegativity
Hydrogen bond acceptors (N: and O:)
Hydrogen bonds will form b/t a molecule that is more electronegative
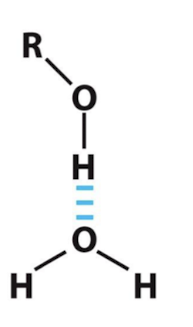
Examples of Hydrogen Bonding b/t Molecules: Alcohol and Water
Water as the hydrogen bond acceptor
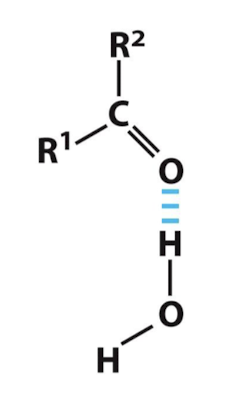
Examples of Hydrogen Bonding b/t Molecules: Ketone and Water
Water as the hydrogen bond donor
Hydrogen Bonds are Directional
Hydrogen bonding is stronger when 3 atoms involved lie in a line
At an angle → Weaker hydrogen bond
Weak Noncovalent Interactions
Hydrophobic Interactions
Displacement of water
Largely formed by dipole b/t 2 hydrophobic molecules
Pi Stacking
Aromatic ring stacking
Regions of opposite changes (polarity)
Van der Waals Interactions
Weak but many
Based on the fact that molecules have multiple dipoles
Hydrogen Bond
Electrostatic
Opposites attract, like repel
Salt Bridge (Hydrogen Bonding + Electrostatic)
Carboxylate amino side-chain (Asp, Glu) to basic amino side-chain (Arg, Lys)
Specifically b/t charged residues, NOT based on dipole, hydrogen bonding, or hydrophobic interaction
Amphipathic Compounds in Aqueous Solution
Amphipathic
Both hydrophilic (likes water, polar / charged) and hydrophobic (dislikes water, nonpolar)
Long-chain Fatty Acids
Hydrophobic alkyl chains surrounded by layer of highly ordered water molecules
Free Energy for Dissolving Non-Polar Molecule in Water
Unfavorable
ΔH = positive
ΔS = negative
You’re going from a high entropy state to low energy state
ΔG = positive (unfavorable)
Dispersion of Lipids in H2O
Each lipid molecule forces surrounding H2O molecules to become highly ordered
Amphipathic molecules will typically come together to form these solid structures
Clusters of Lipid Molecules
Only lipid portions at edge of cluster force the ordering of water
Fewer H2O molecules are ordered
Entropy increase
Micelles
All hydrophobic groups are sequestered from water
Ordered shell of H2O molecules is minimized
Entropy increased
Amphipathic molecules will typically aggregate to form micelles in water
Clustering together in micelles → Fatty acid molecules reduce hydrophobic surface area exposed to water
Fewer water molecules required to form shell of ordered water around hydrophobic surface
Energy gained by freeing immobilized water molecules stabilizes the micelle
Entropy Increase
Many amphipathic molecules stabilized by hydrophobic effects
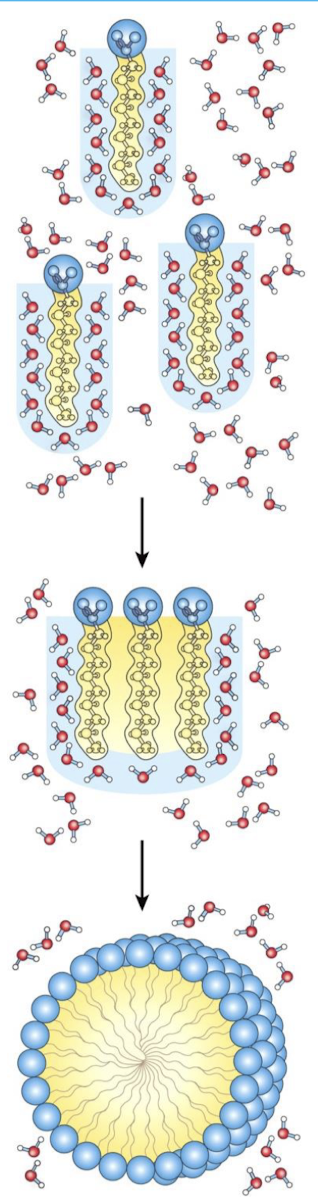
Dispersion of Lipids in H2O
Clusters of Lipid Molecules
Micelles
Effect of Water on Enzyme-Substrate Interactions
Typically, the water molecules around the substrate will disperse and facilitate the formation of hydrogen bonding of substrate and enzyme
Favorable b/c water molecule will coat whatever molecule
Enzyme-substrate interactions stabilized by hydrogen-bonding, ionic, and hydrophobic interactions
Cumulative Effect of Weak Interactions
Macromolecules
Most stable structure usually maximizes weak interactions
H2O molecules often bind tightly to biomolecules that they are part of crystal structure
Water is Partially Ionized
H2O molecules → Slight tendency to undergo reversible ionization to yield hydrogen ion (a proton) and a hydroxide ion
Hydrogen ions are immediately hydrated to form hydronium ions (H3O+)
These