adaptive and innate immunity 2
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
What does the 3rd signal for T cell activation do?
It is related to a soluble molecule that determines which type of cytokine is released by DCs and teh type of subset the T cell will have
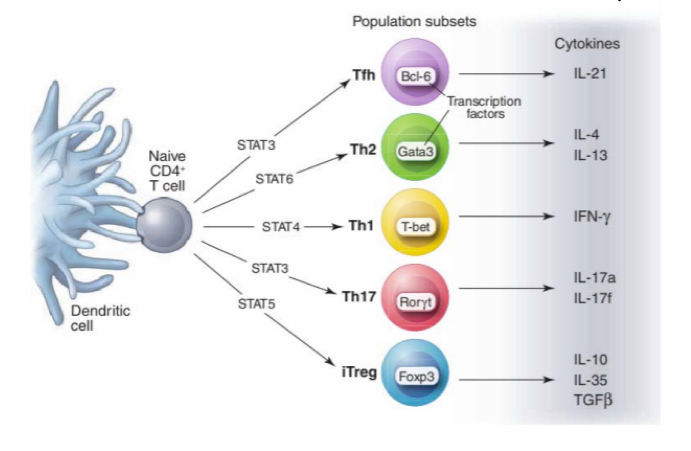
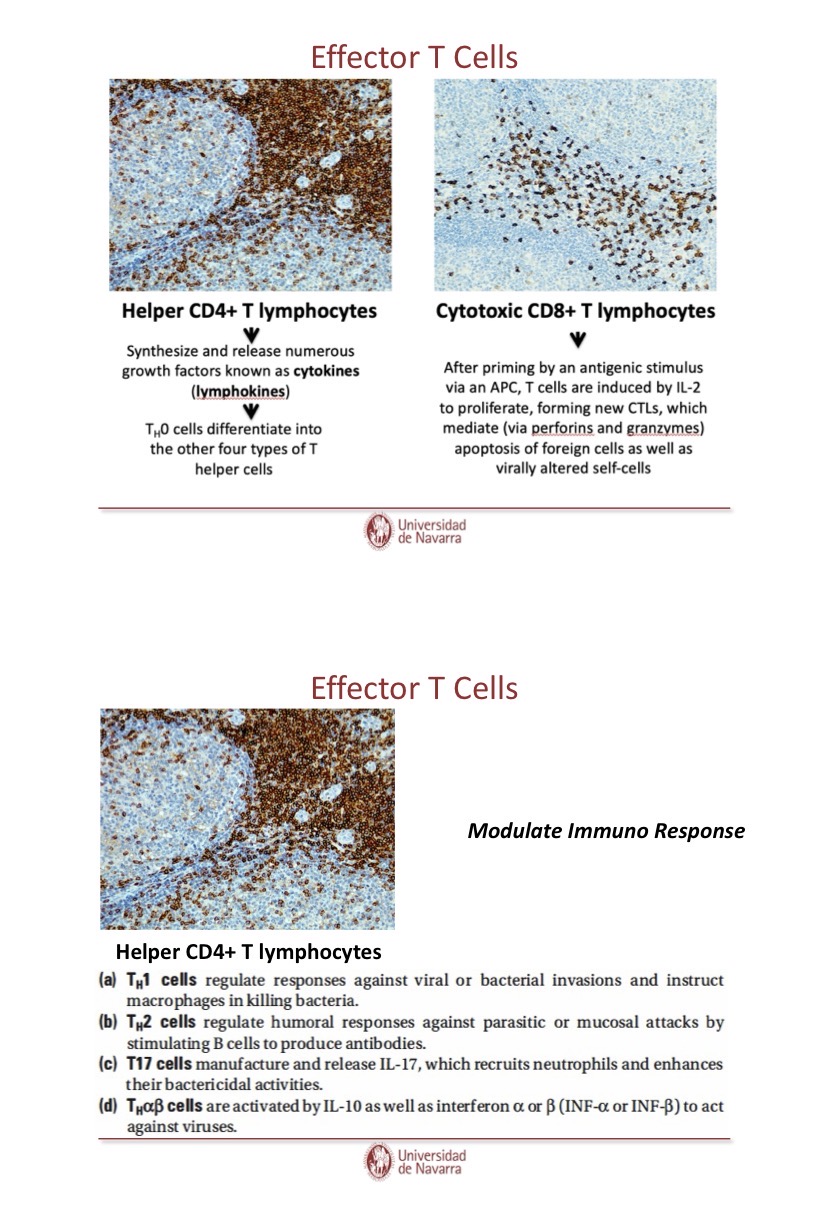
What are Th1 cells?
They produce IFn gamma triggered by the DC release of IL12 (signal 3), also characterized by release of TNF
activates macrophages, nuetrophils, and aid B cells in producing antibodies that will be used inopsonisation.
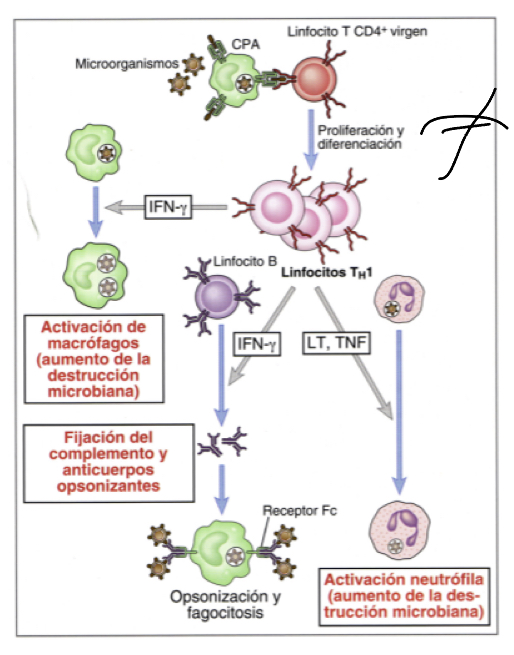
What are Th2 cells?
They produce IL4 (interleukin 4), IL5 and IL10, generally acivated during the rpesence of pathogens that cannot be phagocytosed
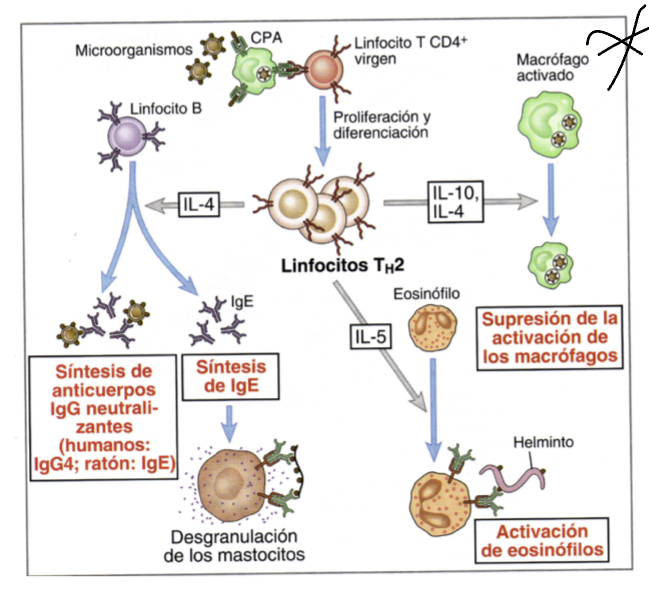
What does IL4 do?
released when multicellular organism that cannot be phagocytosed are identified, they inacivate macrophages, acting on B lymphocytes to carry out opsonization and produce neutralizing antibodie and IgE
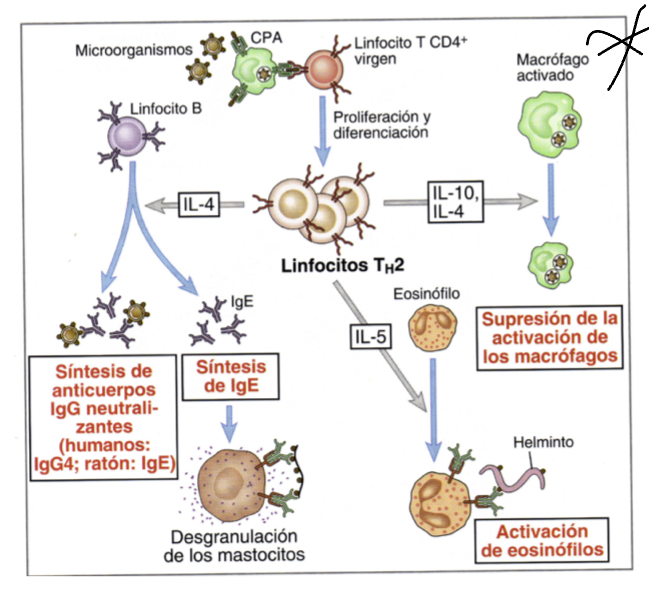
What does IL5 do?
triggers activation of eosinophils whcih can inactivate parasites
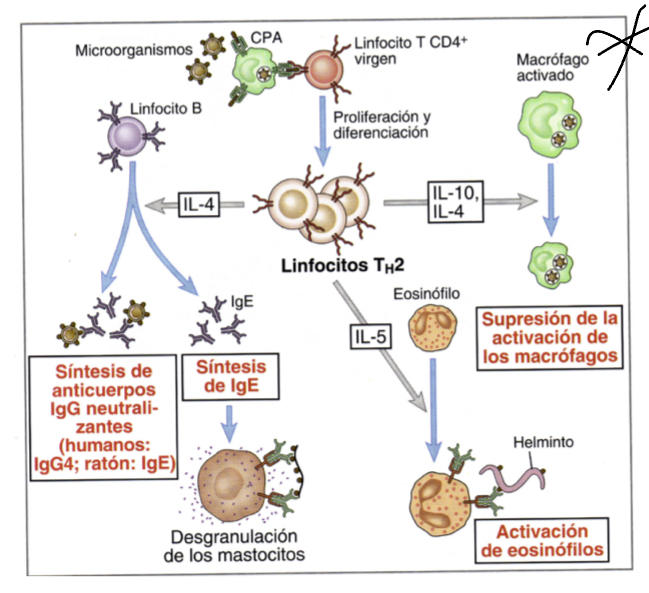
What does IL10 do?
like IL4 released when multicellular organism that cannot be phagocytosed are identified, they inacivate macrophages
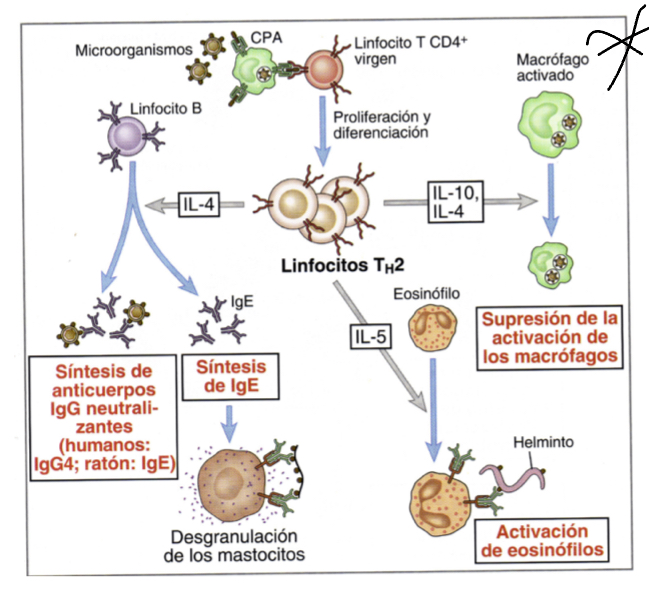
Whatdoes IgE do when expressed by Th2 cells?
induces degranulation by binding to their corresponding ligans on mast cells, these granules will recruit inflammatory factors that induce other responses
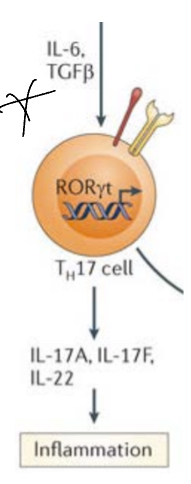
What are Th17 cells?
They produce IL17 which has been described in inflammatory and autoimmune diseases, activated by the presence of IL6 and TGF-beta, IL23 also plays a role in finalizing the subset commitment (regulated by RORgamma-t as it induces IL17 release)
Due to the presence of these cytokines, TFs are expressed inside the T cell which go to the nucleus and induce theproduction of specific cytokines.
Some autoimmnue diseases are caused bu the effect of Th17 cells, thru the expression of IL17 due to the reognition of self antigens can be potenially harmful.
The types of microbes which are targeted by Th17 are mainly extracllular pathogens (fungal and extracellular bacteria)— this si also the main mechnaism to eliminate them.
What are T regulatory cells?
They don’t promote a positive signal, but have a modulatory effect. They’ve come educated from the thymus, undergone negative and positvi selection, howevr the ones prevailing are teh ones with the highest affinity— denominated natural regulatory T cells (because they releases factor that are immunosupressive- contorl the response)
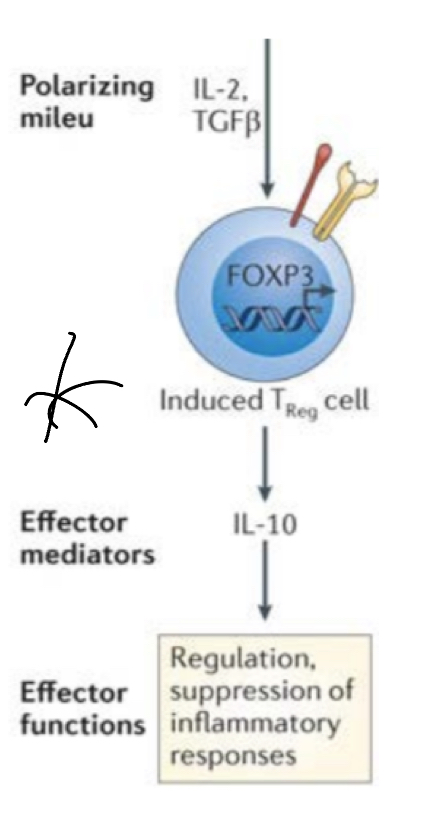
There is another type of Treg cell that doesn’t arise from the thymus that became effector T cell but recieved with signal 3s from cytokines with immunosuppressive/immunoregulatory properties(like TGF beta) that made them induced T cells.
TGF- beta induces FoxP3 master regulator, shifting activating cells into this subset— FoxP3 enters nucleus and triggers transcription of specific cytokines: IL10 (inhibits macrophages) and more TGF-beta (downregukate immune resposne)
downregulates inflammation by inhibit APCs and suppressing other T cell subestets (more Treg generation thru TGF-beta) by producing factors that act at different stages of the immune response.
overall inhibits effector T cell responses
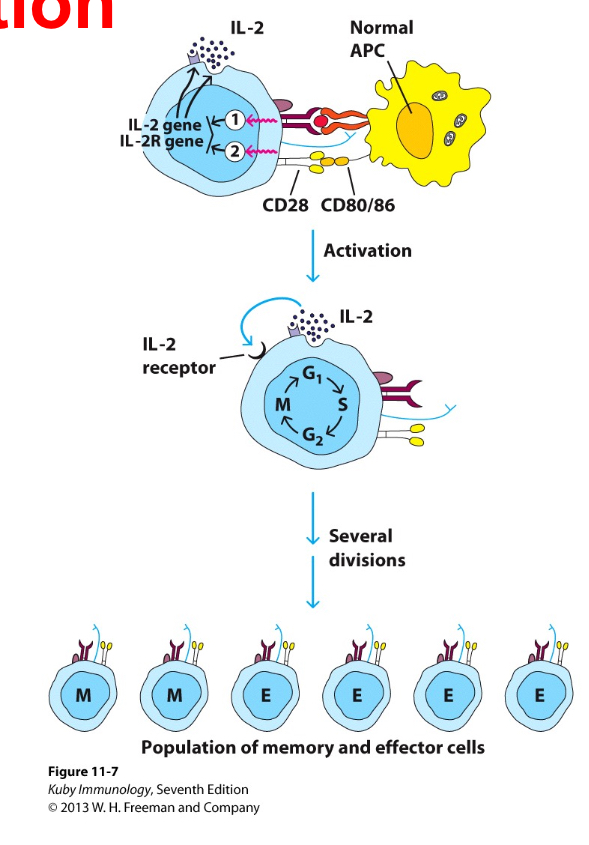
Explain T cell proliferation
Mediated by autocrine growth secretion pathway, the main cytokine for proliferation is IL2. T cells produce cytokines, expres receptors for these cytokine and respond to the binding of these cytokines by proliferating— so IL2 will act on the cell which release it, inducing its growth and not the growh of oter sets of T cells.. hence it can be used as a marker for T cell activation.
As a consequence, from a few naive T cells many effector T cells (many more produced) and memory T cells (not as many produced) are generated, once the pathogen is cleared the number of cells is reduced and only those with memory function wll remain.
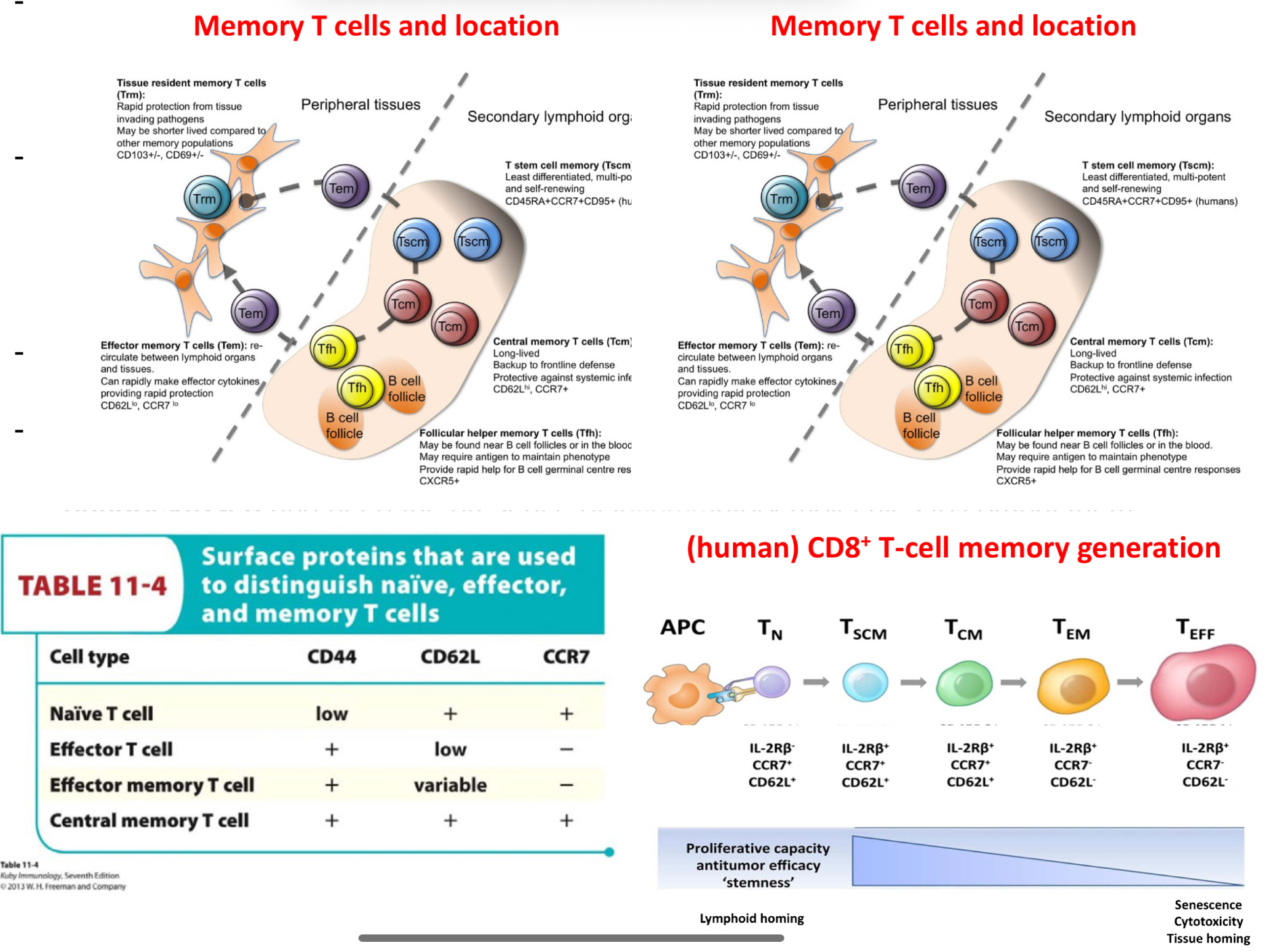
Explain T cell differentiation into memory cells
Memry T cells persist even in the absence of pathogens, this is due to homeostatic cytokines which manage to maintain the memory cells alive. For example CD8 cells depend on IL15 for the generation of emmory cells.
The CD44 mans it is no longer naive T cell, likely has come into contat with antigens.
CD62L (selectin) (in secondary lymphoid organs)- naive cells and central memory cells in 2ary lymphoid organs will express them
CCR7 it is a chemokine.. which will make cells gravitate towards it— if cells have receptors for these chemokines, they will inevitably migrate towards them( found in secodnary lymphoid organs)
***hence naive T cella dn central emmory cells will be brought to these seocndary lymphoid organs from their attraction to chemokine CCR7.
(TABLE )- naive in lymphoid rgans, effector T cell in places were needed (pahtogen locations), effector memory T cellswill be everywhere in the organs, in cas eth antigens is found, centralmemory t cells are are liek naive T cells but more ready than them for anitgen recognition and then effcetor function in lymphoid organs.
Explain the downregulation of the T cell response
excess of effector cells no longer necessary… no more anitgens, co-stimulatory molecules (no signal 2), and cytokines induced by inflmmation are produced— hence these is nothing to actvate the T cells.. they die due to homeostatic mechanism and leave a lower memory T cell numbers.
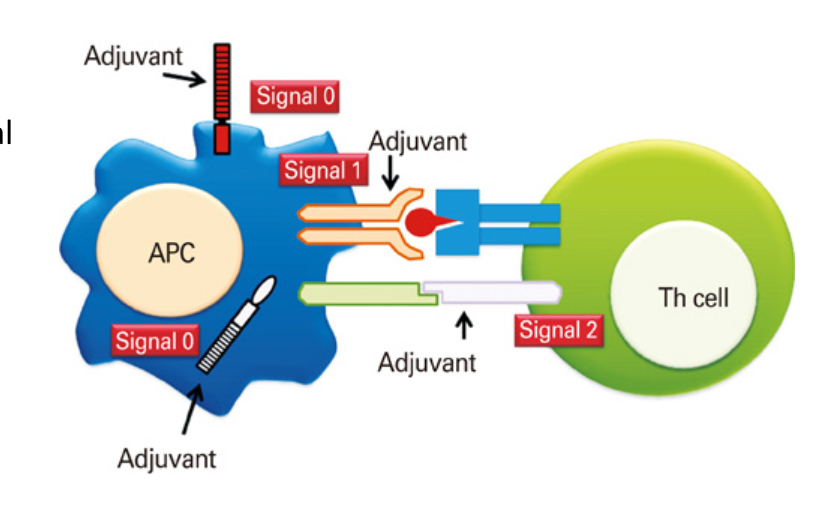
Explain the importance of co-stimulatory molecules in vaccinations
T cell triggering requires siganl 1 and 2 by APCs but vaccines lack PAMPs to sitmulate signal 2, therefore additional ADJUVANTS need to be added to induce the activation of signal 2
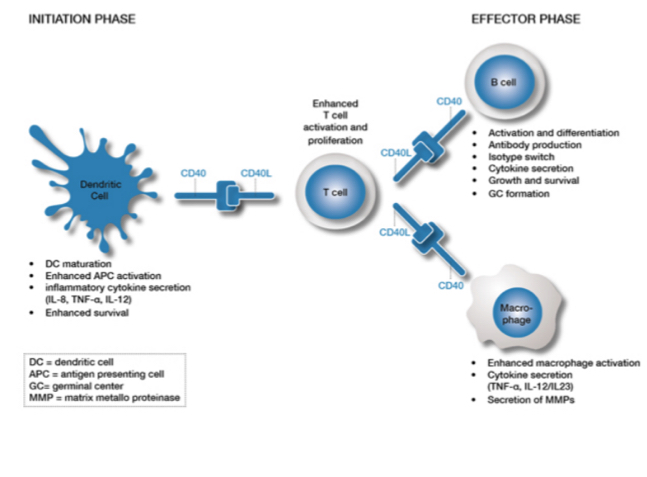
What is CD40 and CD40L in T cell activation?
CD40L is a ligand expresed when t cells are activated, they upregulate the T cells, CD40L acts on CD40 (found on APCs) to reinforce presentation, The activated Tcells can respond to APCs and they CD40L will causes a stronger stimulation of the APC… additionally, CD40L and CD40 actiavtes B cells and macrophages.
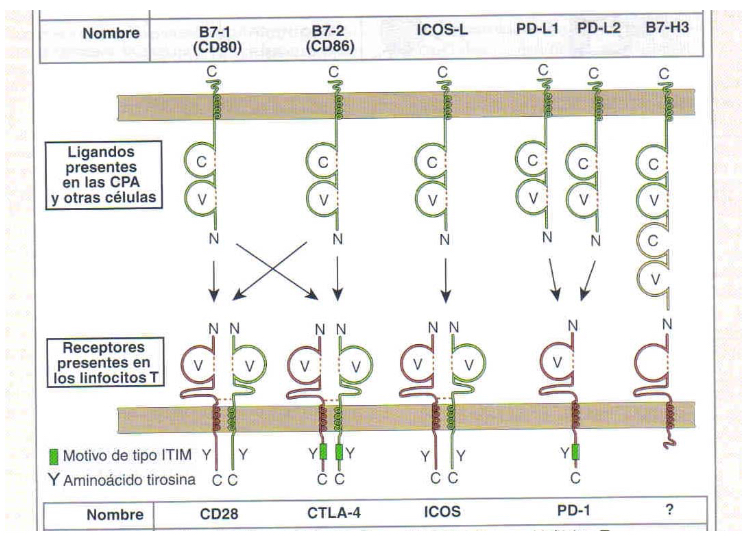
describe the general features of B cell responses
at first they dont secret antibodes, only do so as B cell receptors ont heir membrane.. there are T cell dependent B cells (antibodies against a protein anitgen) andd T cell indepedent B cells ( anitbodies aganst non-protein antigen)
explain T cell dependent B cell activation
antigen recognition thu BCR in addition to the recruitmnt of T cells for additional activator signals.
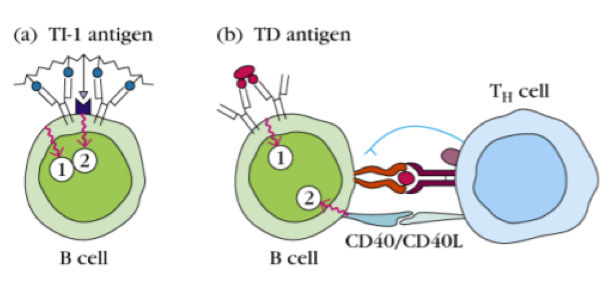
explain t cell independent B cell activation
(basically bypasses the need for T cells by causing stronger or additional signalling)
type1: induced by type 1 antigens, like LPS (which is a PAMP and can be rcognized by a Bcell with TLR4 and a specific immunoglobulin for LPS)— by receiving these tow signals, the B cells can be independent from T cell elp.
Type 2: type 2 antigens which are polymeric (repetition of some structure) (like polysaccharides or flagellin), so instead of single differnet epitopes, we ave many repititions of the same epitpe, hihc cause sthe signalsent in to be much stronger han when ecognizing a classical protein.. this alows teh B cell to be activated withou T cell help.
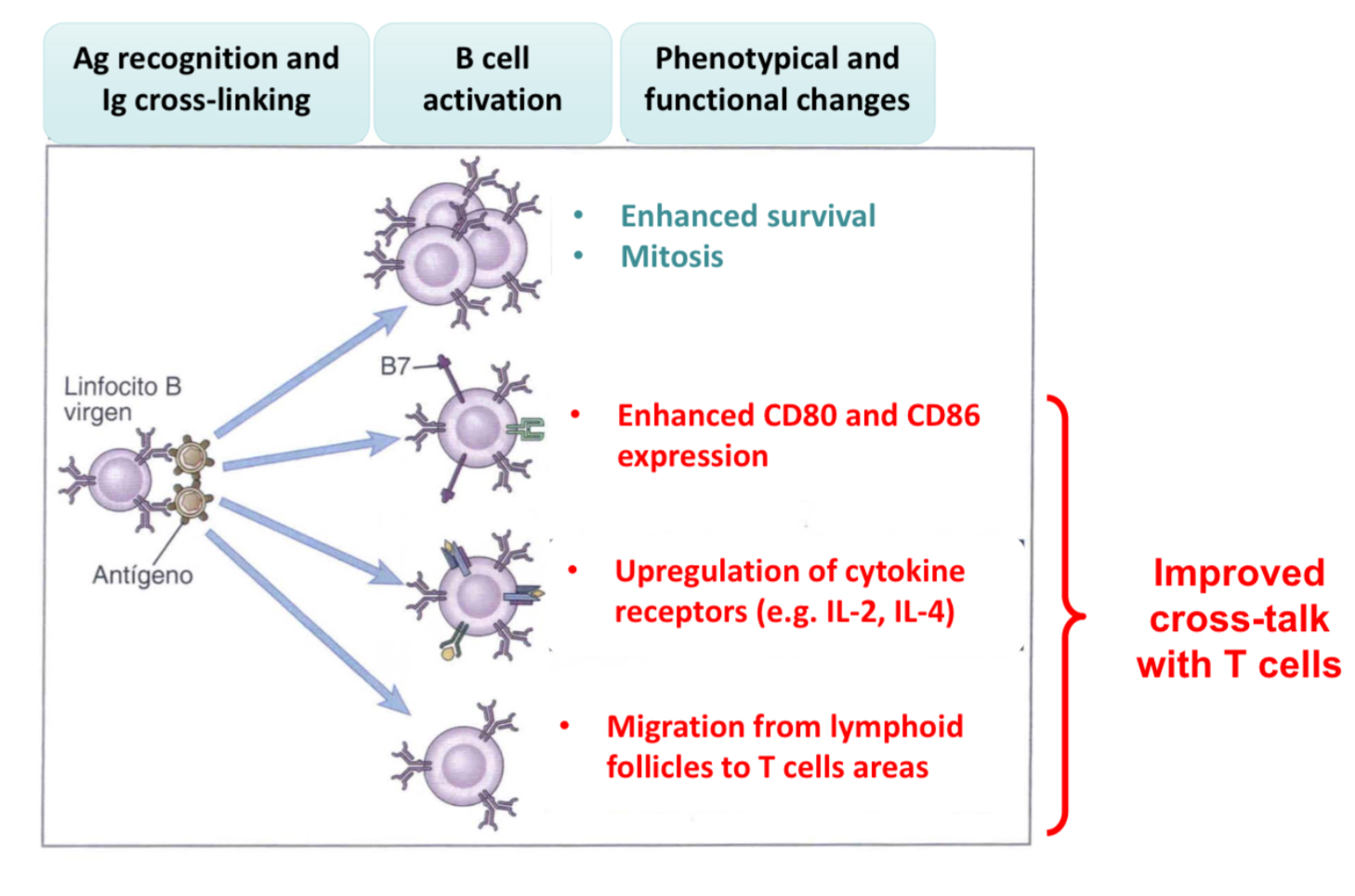
Explain what the functional response induce by anitgen rcognition are for B cells?
start proliferating
Expres CD80 and CD86 (B7 family), thus providing ligans for B cell signal 2 and acting as co-signaling molecules
Upregulation of cytokine receptors by relasing cytokines
B cells migrate to T cell areas wihtin lymph nodes to interact.
These changes will cause thenaive B cell to recognize the antigen thru Igs and uptake it to become an APC, presneting the antigen on MHCI or MHCII.. therefore it can induce signal 1 and 2 in the T cell.
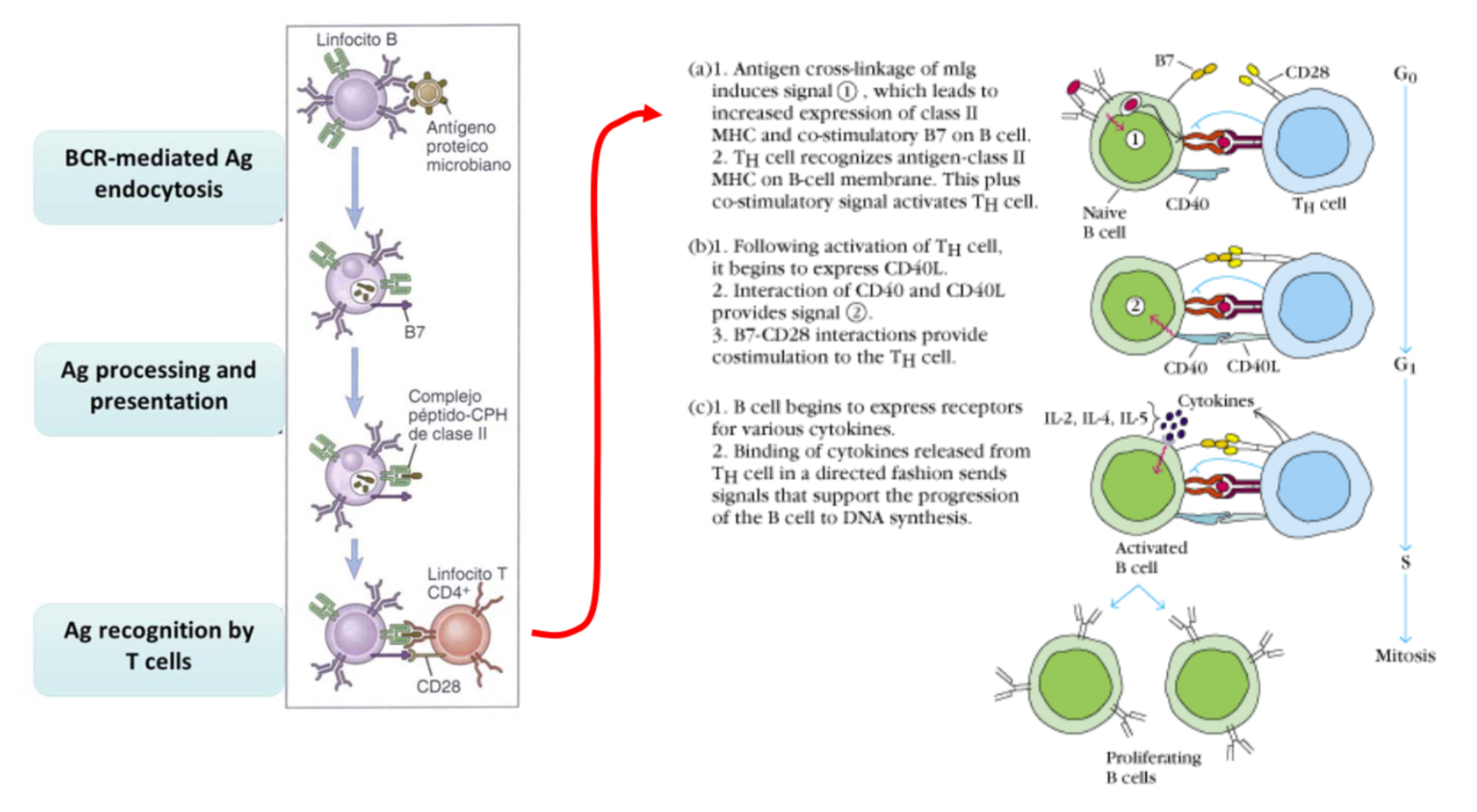
What is the role of T cells in humoral response?
T cells express CCD40L and soluble cytokines, B cells express CD40, the T sends a signal to the B cell which is presenting the antigen and so the B cell can present the receptor for cytokines and so proliferate upon bidning to these cytokines.
What are the biological consequences of anitgen presentation of B cells to Th?
Linked recognition: there is a linked recognition since both B and T cell epitopes must be on the cells in order for the signal to be transmitted. Haptens have B but not T epitope or oposite way, meaning there is blocking of mchenaism. Immunogens hence have both B and T reactant to induce response.
Coordinated responses: in refernece to linked recognition, the T and B cells responses are tehrefore coordinated
minimal antigenic size necessary since it must contain a B cell epitope and a T cell epitope
B cell responses are MHC restricted , and so by consequence so are t cell responses
reduced likelihood of autoimmunity: self antigen recognizing cells are eliminated, however, if some escape this selective mechanism, both the T and B cells must have escaped the selection in order to causes autoimmunity.
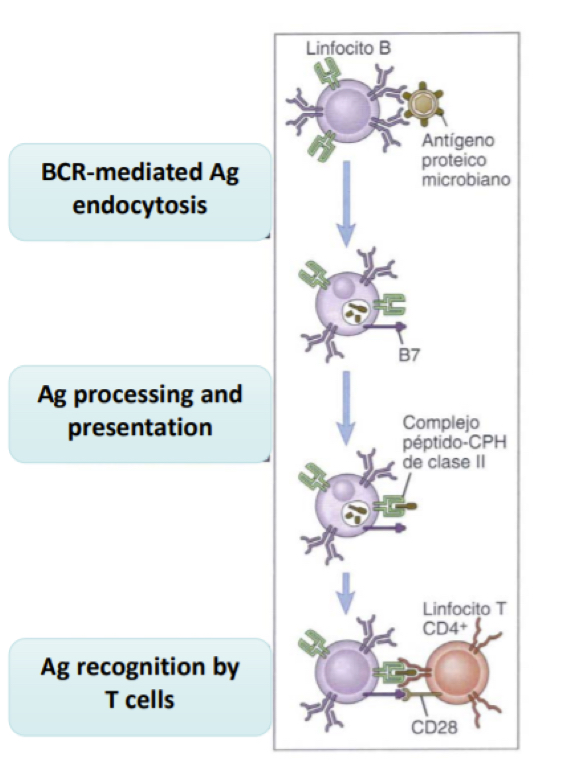
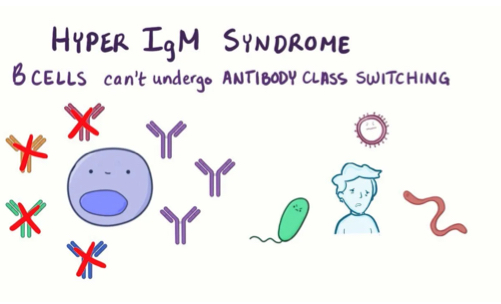
What is the roe and importance of CD40 and CD40L in B cell activation?
CD40L and CD40 are necessary for isotype switching which is necessary to allow the sleection of specific isotypes for specific infections.. if this becomes mutated it is known as the X-linked hyper IgM syndrome which is.. characterized by high levels of IgM, but lacking IgG and IgA and makes people suscetible to bacterial infections.
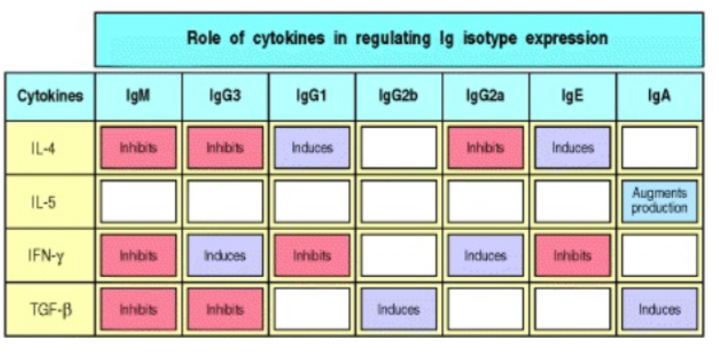
Explain cytokine role in B cells responss
induce proliferation (quantiative effect) and isotype switching (Qualitative effect)- dictate which isotype is produced since anitgen binded to will determien this
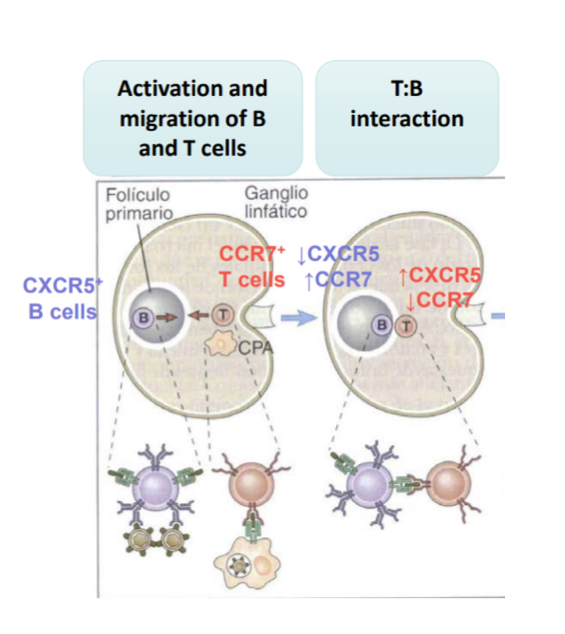
Explain the initiation immune resposne by the of T and B cell interaction
initially T and B cells are at diff locations, once B and T cells recognize the apthogen antigen, their chemokine receptors change and so they migrate elsewhere, meeting eachother along the way.. the B cell will begin to secret antibdies and isotype switching
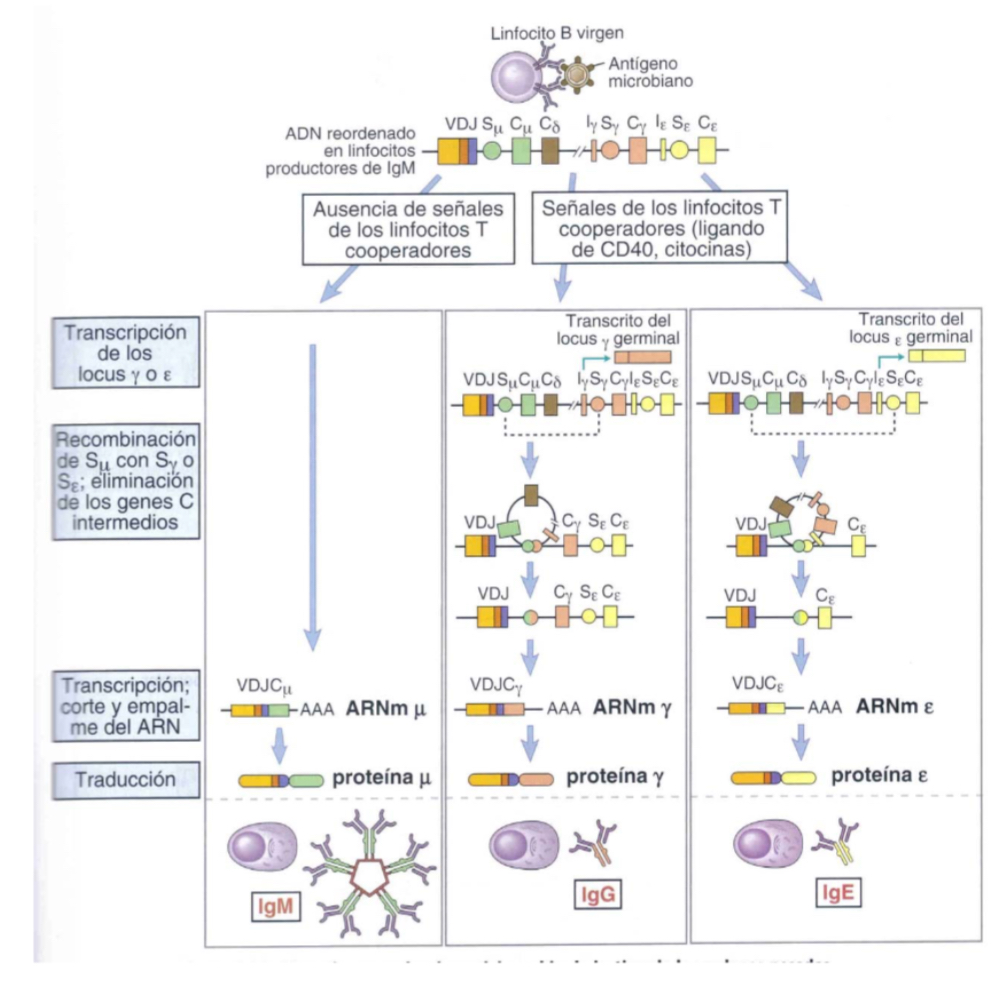
Explain B cell differentiation into plasma cells
Upon recieving a signal for activation, further processing is induced and Ab secretion s promoted by CD40L and cytokines like IL2, IL4 and IL5.. however these Ab now lack the intramembrane tail and become soluble.. shorter heavy chains= secretion by ciculatng B cells or longterms Bcells in the bone marrow.

Explain the class switching of heavy chains inB cells
B cells express IgM and the IgD, however in presence of cytokines, other isotypes will be reduced… loops are established betwene S region nd the S of the constant region gene of another isotype, th eloop is eliminated but the first region is now one that codes for another gene.. a new Ig wiht different C region wll be produced.. a bigger loop can be made to produce a differnet Ab, hoever the variabe region VDJ remains unchanged, so specificty is the same.
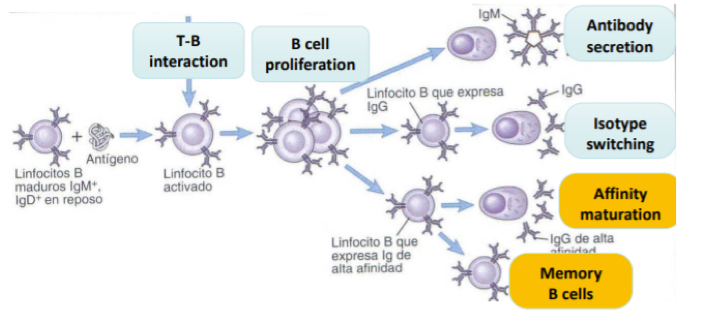
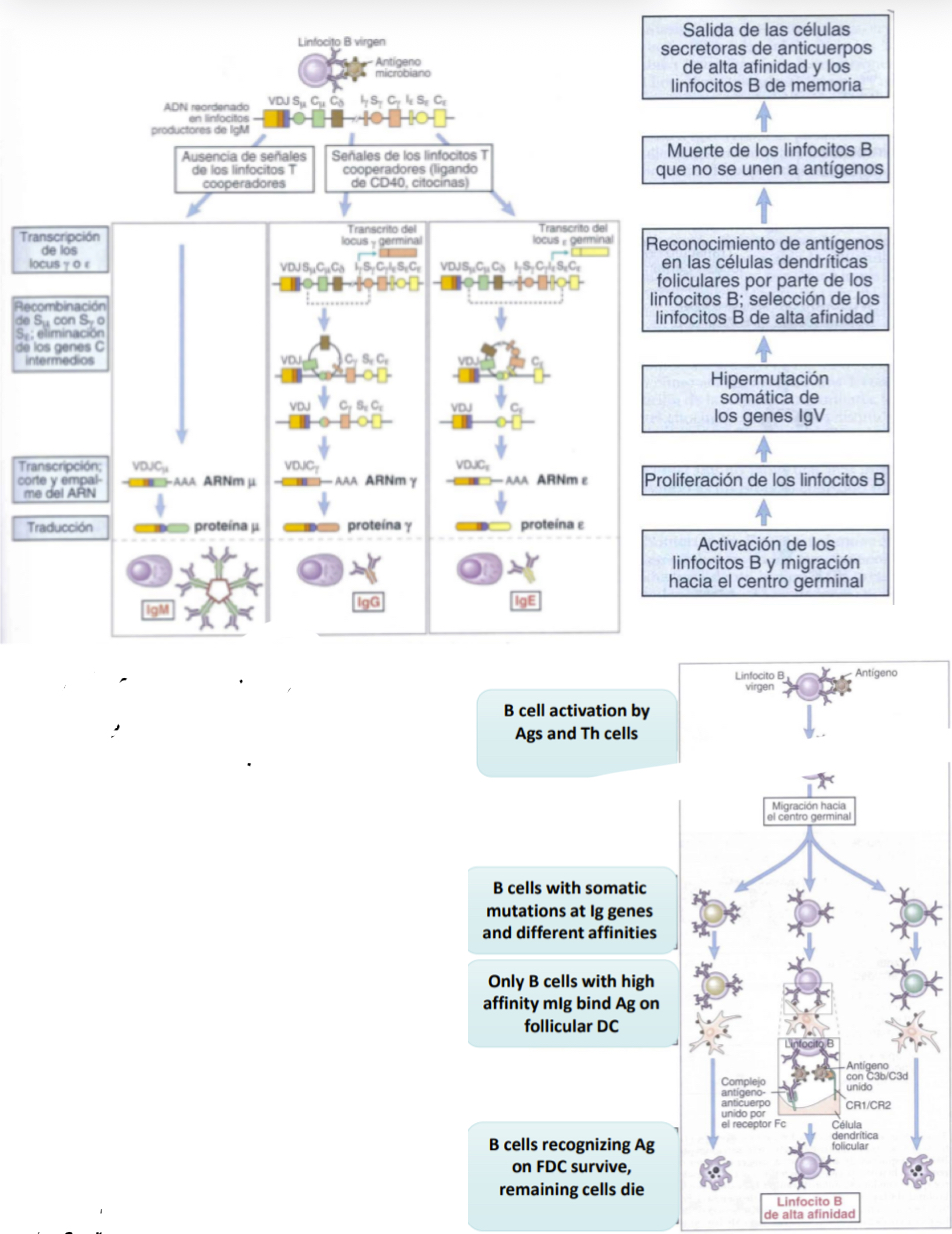
What is afinity maturation?
Each time a B cell multiplies it undergoes signalling and so mutations, eahc egenration the B cells get more or less or the same affinity… eventuallythe B cells with the most affinity to antigens are selected for and the rest die. Follicular dendritic cells aid in this as they present teh antigens that will help the seection of these B cells. The B cells slected for wll continue to become memory cells.
Explain B cell memory responses
The seocnd time recognizing the ntigen, the esponse is much quicker, stronger (more porduced) and qualitatively, the isotype of the Abs wll be different— IgG or IgA instead of beggingin IgM… since B cells have been able to have affinity maturation, they will work much better (since they have higher affinities).
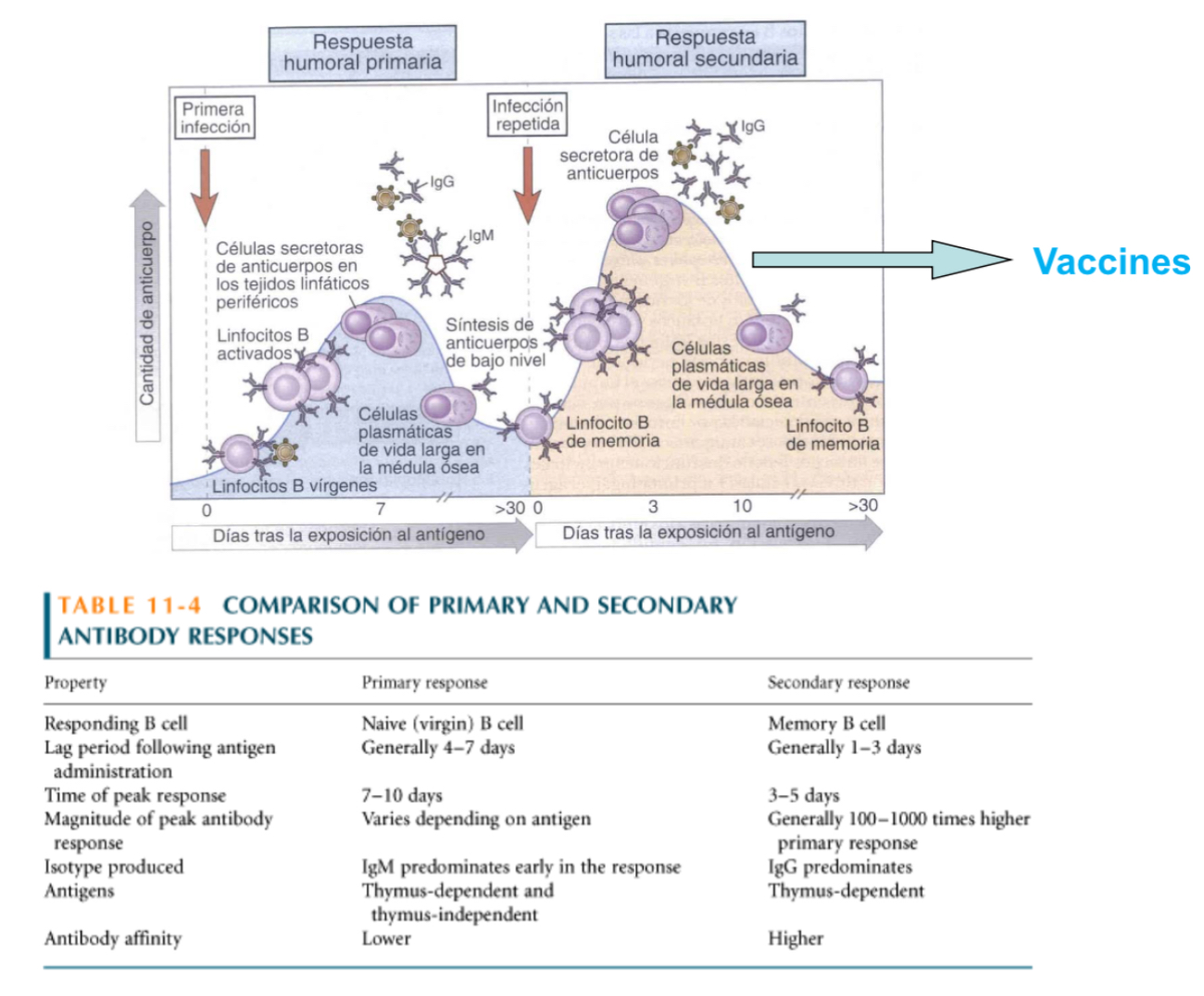
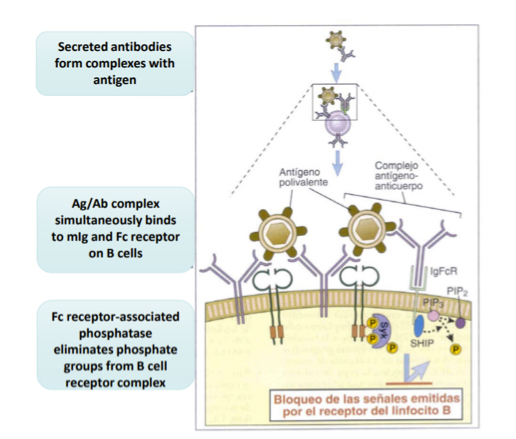
Explain the regulationof the immune response in B cells thru Fc receptors
As respons progresses, high amount of Ag-Ab complexes form. B cells have BCRs which will bind to the antigen and the Fc (constant regions) will bind to the antibodies.. since the Fc regions is connecte dintracllualryto phsophatases, they will begin to dephosphorylate and downregulate phosphate groups from BCRs which complexes to stop antibody production.
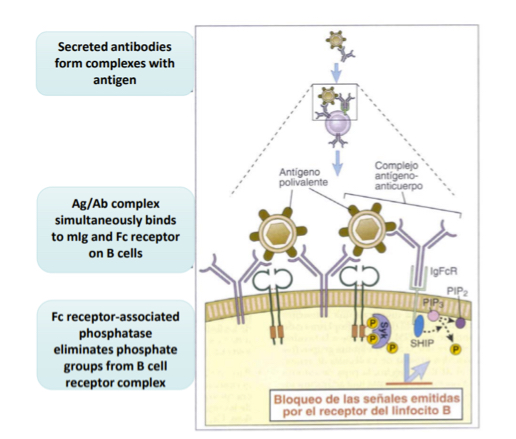
Explain the genral features of lymphocyte activation T cells vs B cell
T cell activation: require anitgen recognition from APCs and co-stimlation(CD80 and CD86) and cytokines (from DCs)… in short, signals 1,2, and3
B cell activation: recognize free of cell associated antigens in their native form.
Explian th epathways by which T cell anitgen recognition is aided.
SInce BCRs have a higher affinity than TCRs.. TCRs need more signalling
multiple receptor crosslinking upon anitgen recognition causes phosphorylationwhich tirggers enzyme and activation and adaptor recrutiment.. these will activate several main signalling pathways which lead to the transcription factors in the nucleus to produce Abs.
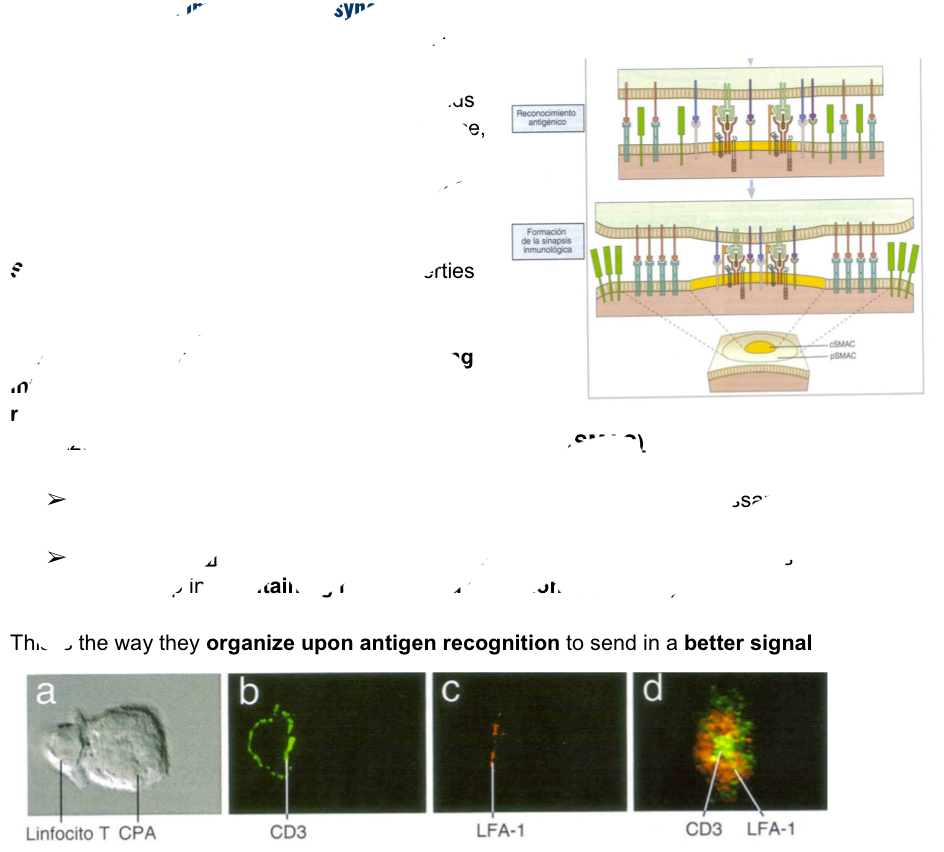
Explain what he immunological synpses is as the beggingin of T cell anitgen recognition.
Integrins allows cell-cell interactions with the cytoskeleton, when integrins proeprites are modified, the cytoseleton willbe reorganized and will end up a the orgamnized structure: supraolecular activator complex (SMAC) and can be one of two ways:
Central SMAC: found in center providing all molecule snecessary for anitgen recognition and signal (ex: CD3)
Peripheral SMAC: found around the center, composinng the ring of molecules which maintain binding and adhesion (like LFA 1)
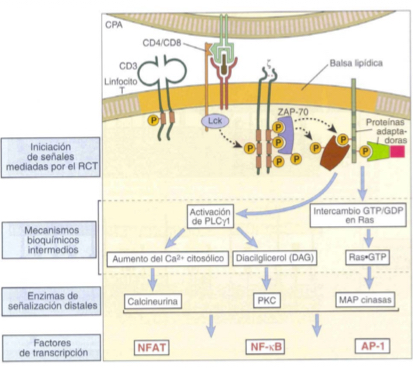
Explain phsophorylation in T cells activation
Lck becomes activated and phosphorylates theITAMs of CD3 and he zeta chain
Changes zeta chain’s shape and a molecule known as ZAP-70 docs into the ITAM regions, carring out phosphorylation and autophosphorylation.
ZAP-70 phosphorylates other molecules like LAT so that teh cascade can begin
Describe the recruitment of adaptor molecules as part of T cell activation
adaptor molecules combine phosphorylated molecule swiht additional enzymes
In this way initial proteins can be connected to the first wave of enzymes to trigger the cascade— in t cell activation for example: LAT connects other adaptor proteins and enzyes like PLC gamma-1
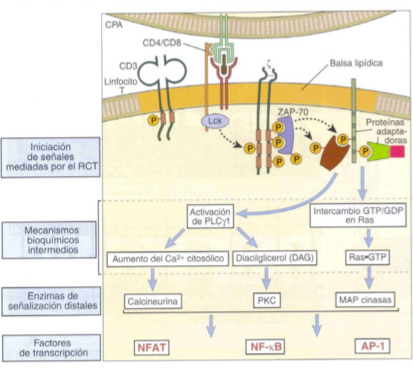
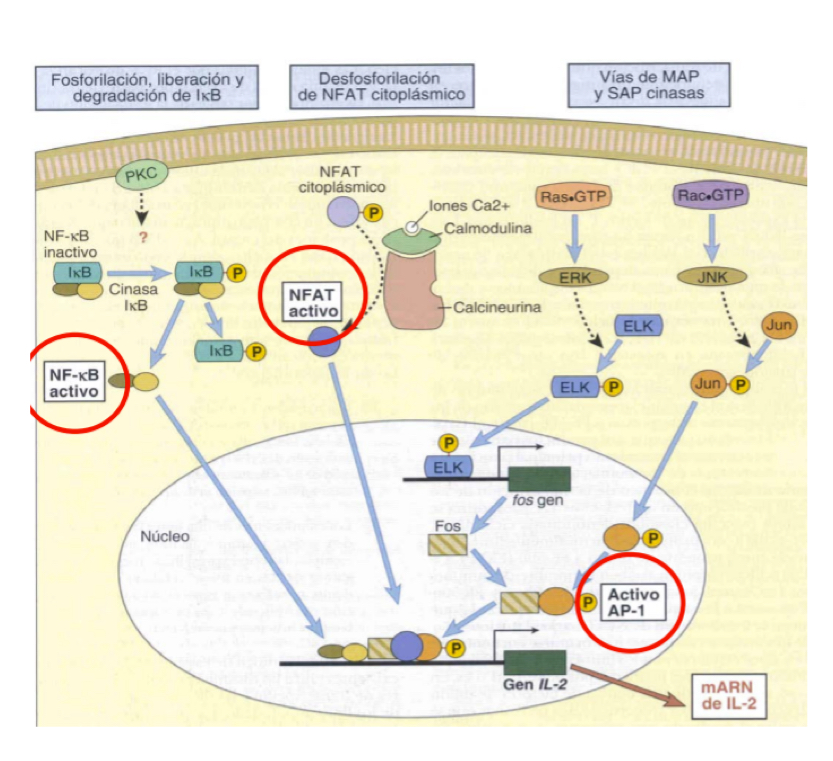
Describe the Ras-MAP kinase signalling pathway in T cell activation ***MUST KNOW FOR TEST**** (AP-1)
LAT phosphoylation
Adaptor proteins like Grb-2 are recrutited and allow binding to SOS
Ras-GDP to Ras-GTP
MAPK cascade+ Ras-GTP
Activation of AP-1 transcription factor
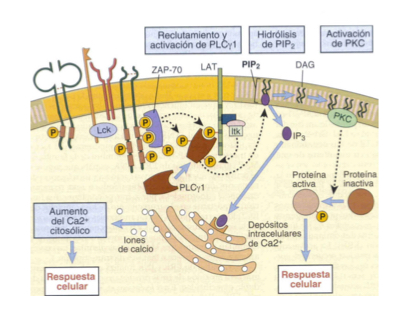
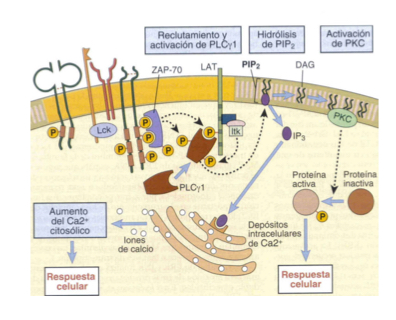
Describe the signalling pathways thru Ca2+ and PLC**MUST KNOW FOR TEST**** (NFAT)
LAT phosphorylation
PLC hydrolizes PIP2 into DAG and IP3
IP3 binds to ER, triggering release of stored Ca2+
Increasee of Ca2+ in cytoplasm- atiavtes a phosphorylase which eiminates P from NFAT
NFAT activated as a transcription factor— necessary for cytokine expression (IL2, IL4, TNF-alpha, etc.)
Describe the activation of transcription factor NF-kappaB**MUST KNOW FOR TEST****
NF-KappaB and I-KappaB are found forming a complex which is inactive. I-kappaB becomes phosphorylated and separates from NFkappa-B which is activated when free and translocates to the nucleus to trigger transcription- essential for cytokine synthesis.
List some immunosupressant for T cells that target the signalling pathways
cyclosporine: inhibits calcium binding
Antibodies: IL2 induces proliferaion, many antibodies can block the IL2 rceeptor
Drugs
Antimetabolites: inhibiting synthesis of purines and blocking cell cycle
Inlammatory mediators: acting on APCs to avoid anitgens presentation and reconition.
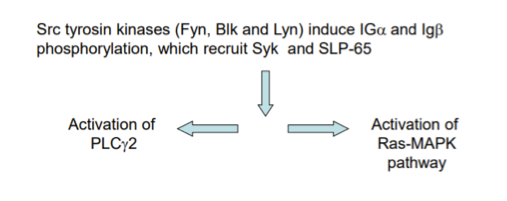
Explain the signal tranductino pathway of B cell activation
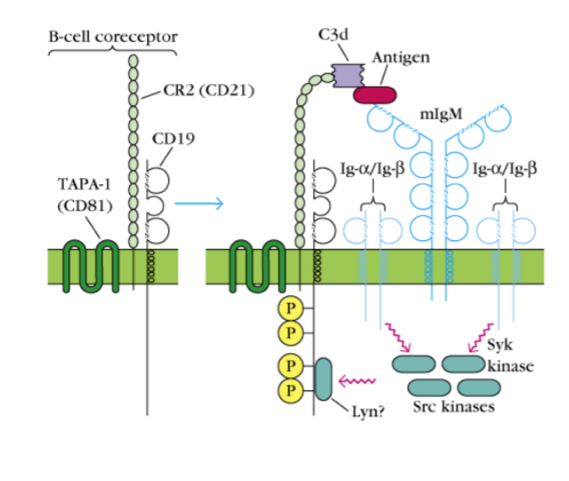
after Ag recognition/binding, Src tyrosine kinases phosphorylate ITAMs of I apha and Ig beta
Syk and SLP-65 bind the phosporyated chain
Following this it is mostly like T cell pathway, excpt PLC gamma 1 from T cells is actually PLC gamma 2 in B cells.
What is the B cell complement receptor?
A receptor that acts a costimulation which reinforces a signal, an atigen an be bound by a BCR and a complement receptor so signals come from both which amplifies it. Hence, antigens that are able to bind complement are much more immunogenc, as they are able to trigge two signalling pathways at the same time.