Properties of periodic groups and elements
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
By the late 1800s, scientists recognized that certain _______ looked alike and ___________ in similar ways
elements, behaved
Dmitri Mendeleev (1834-1907)
arranged the 60 elements known at that time into groups with similar properties and placed them in order of increasing atomic masses
group
Vertical column in the periodic table
group number
A group number is written at the top of each vertical column (group) in the periodic table
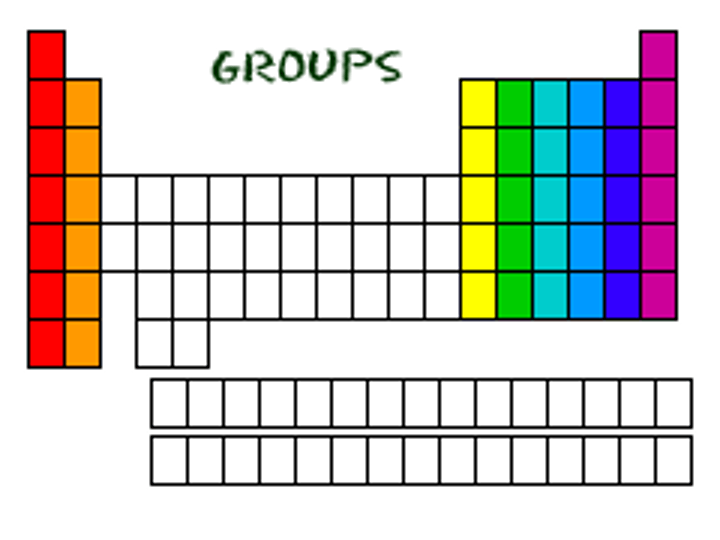
representative elements
an element in an "A" group; as a group these elements display a wide range of physical and chemical properties. In their atoms, the s and p sublevels in the highest occupied energy level are partially filled
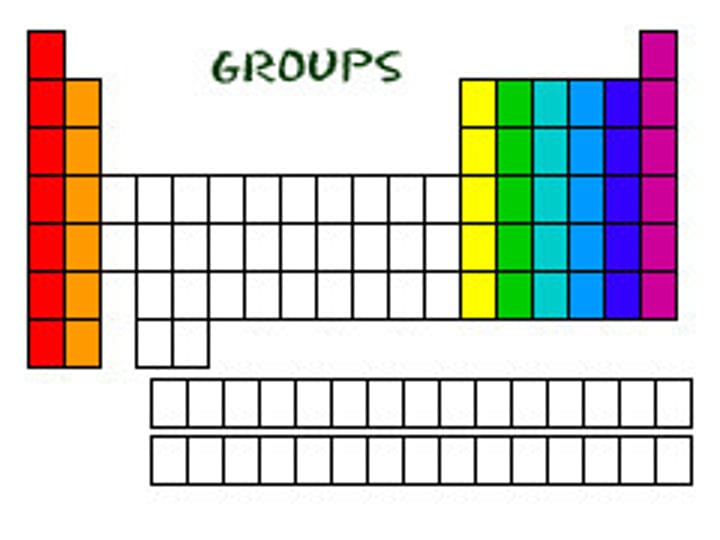
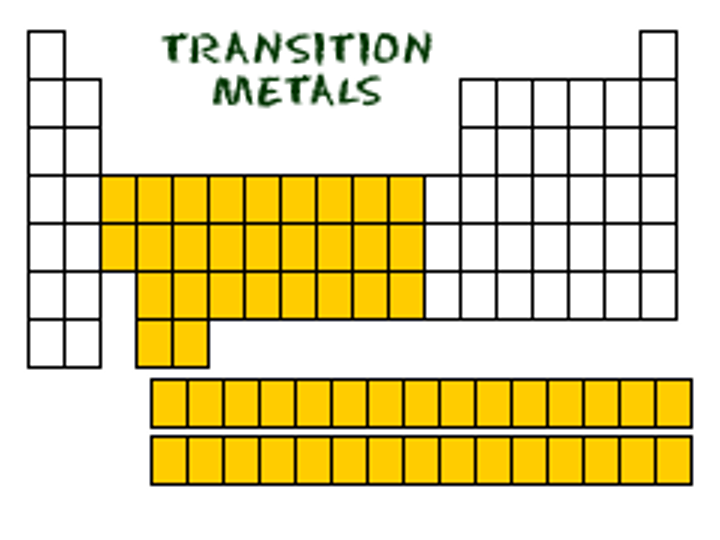
transition elements
an element in the center of the periodic table that is designated with the letter B o the group number 3-12
period
A horizontal row of elements in the periodic table
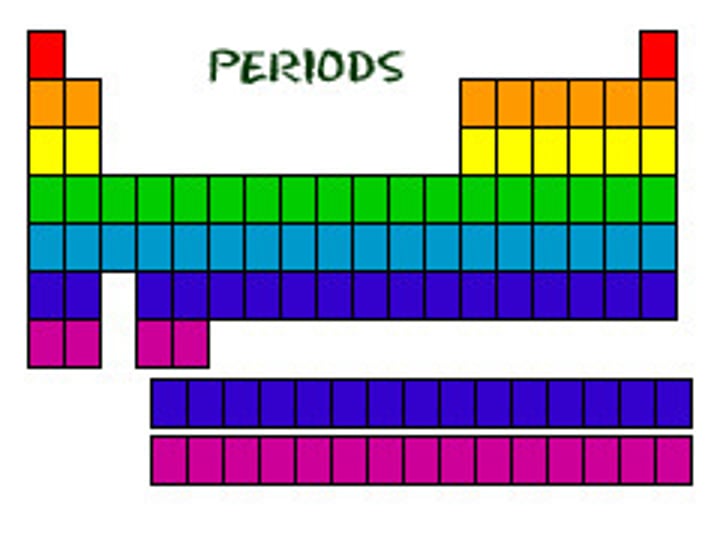
period are counted from
1-7
several groups in the periodic table have _______ _____
special names
group 1A (1) are...
alkali metals
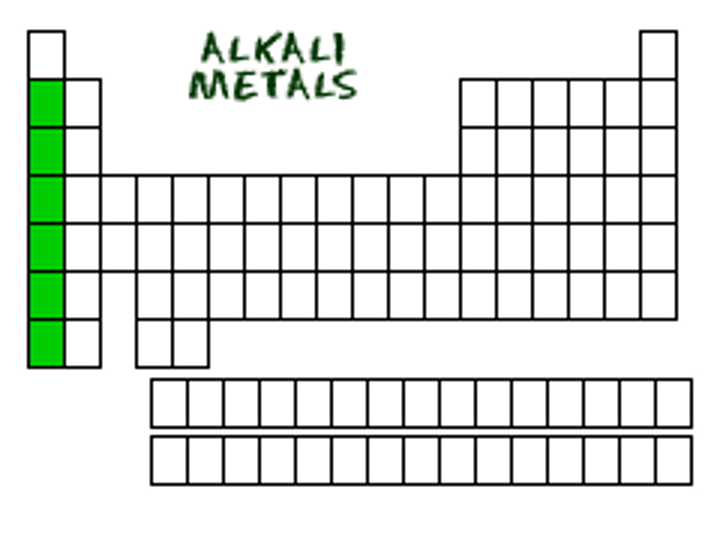
alkali metals
soft, shiny metals
good conductors of heat and electricity
relatively low melting points
react vigorously with water
form white products when combined with oxygen
_________ is not included in the alkali metals
hydrogen
group 2A (2)
alkaline earth metals
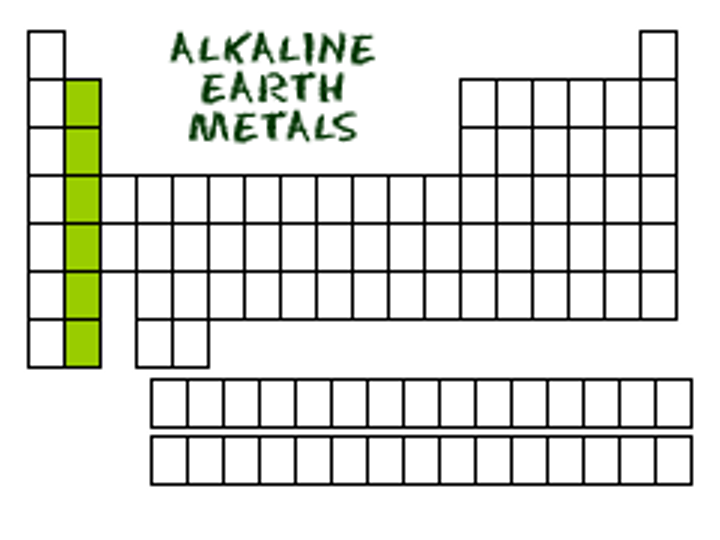
alkaline earth metals
shiny metals
not as reactive as alkali metals
group 7A (17)
Halogens
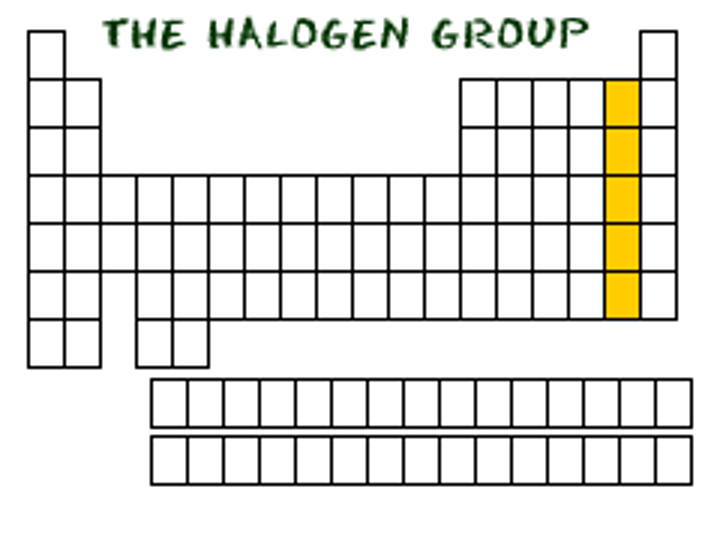
halogens
highly reactive
form compounds with most of the elements
Group 8A (18)
noble gases
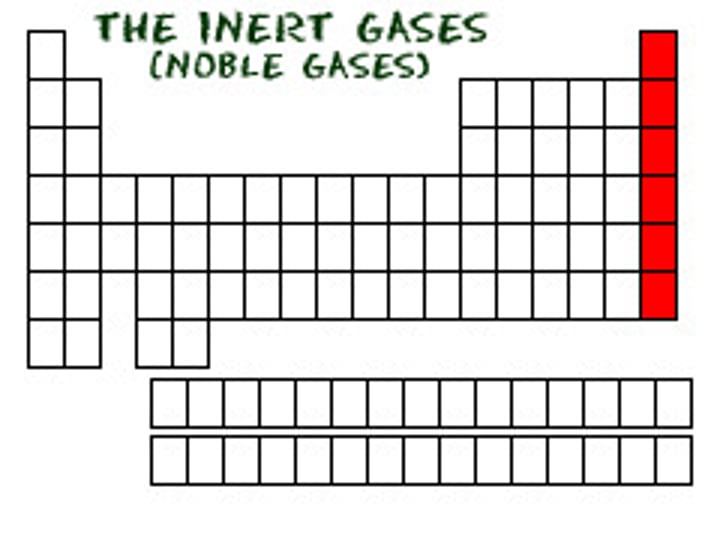
noble gases
unreactive
seldom found in combination with other elements
Another feature of the periodic table is the heavy ________ ___ that separates the elements into the metals and the nonmetals
zig zag line
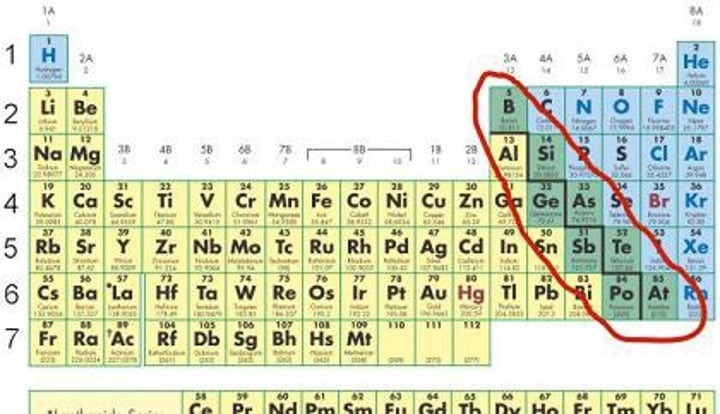
Except for ______, the metals are to the ___ of the line with the nonmetals to the _______.
hydrogen, left, right
metals
shiny solids
ductile (shaped into wires)
malleable (hammered into a flat sheet)
good conductors of heat and electricity
higher melting point than nonmetals
solids at room temperature, except mercury (Hg), which is liquid
nonmetals
not especially shiny, ductile, or malleable
poor conductors of heat and electricity
typically have low melting points and low densities
the elements located along the heavy line are _______
metalloids
Metalloids
have properties of both metals and nonmetals in some respect