pchem exam 3
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
While all mass spectrometers can be used to identify unknowns, which of the followirg are best suited for this purpose?
TOF, triple quad, AMS
When the urine of patients dosed with imatinib is analyzed using a radioactivity detector, which of the following are true?
B. The peaks are broad because of the large detector volume
C. We detect glucuronide and glutathione adducts
D. Oxidative and degradative metabolites are detected
true or false:
A strong cation exchange column is negatively charged
true
Which statement correctly completes the sentence?
The average molecular mass of a compound.....
is the average of the individual isotopic molecular masses corrected for their natural abundances
Fill in the blanks in the correct order...
In HPLC, as the particle size—,the plate height—and the plate number—,leading to an—,in efficiency.
decreases, decreases, increases, increase
True or False:
A degasser in an HPLC system is used to more thoroughly mix the solvents.
false
Oxytetracycline under positive ionization gives an ion of mz 461 (100%) and 483 (14%).
When m/z 461 was subject to fragmentation, it gave an ion at m/z 444 and 426.
What information does this tell us about the molecule?
m/z 444 results from loss of ammonia, m/z 426 results from loss of ammonia and water, the parent molecule has either zero or an even number of nitrogens, it forms a sodium adduct
The full van Deemter equatior contains variables that attempt to quantify the various factors that affect band broadening.
These factors are which of the following?
Eddy diffusion, longitudinal diffusion, equilibration time, linear velocity in cm/s, particle diameter, coating thickness, analyte diffusion
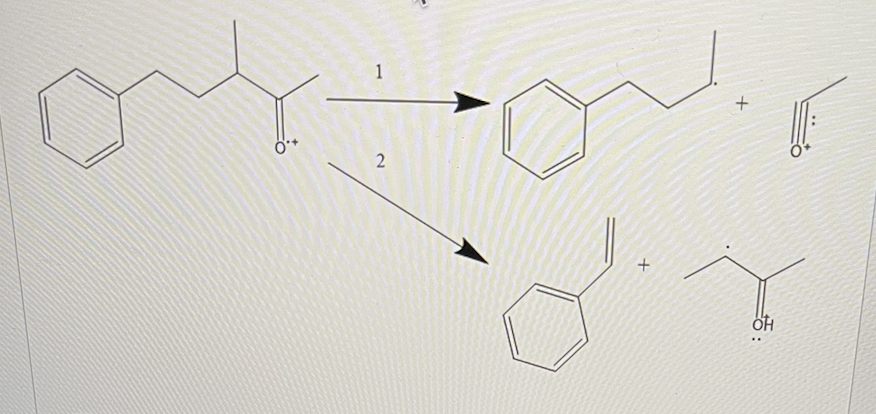
In the reaction mechanism shown below, pathway 1 is an example of _, while pathway 2 is an example of _
Homolytic cleavage, McLafferty rearrangement
True or False:
In mass spectrometry, homolytic cleavage results in an uneven distribution of electrons.
False
For reversed phase chromatography, which is the proper order of increasing polarity?
tetrahydrofuran < acetonitrile < methanol < water
IR provides information on functional groups in a molecule.
True
When a compound with a pKa of 5 is analyzed on a reversed phase HPLC using a buffer with a pH of 6.5,.....
It will elute faster off the column than with a buffer of pH 4 because it is mostly deprotonated
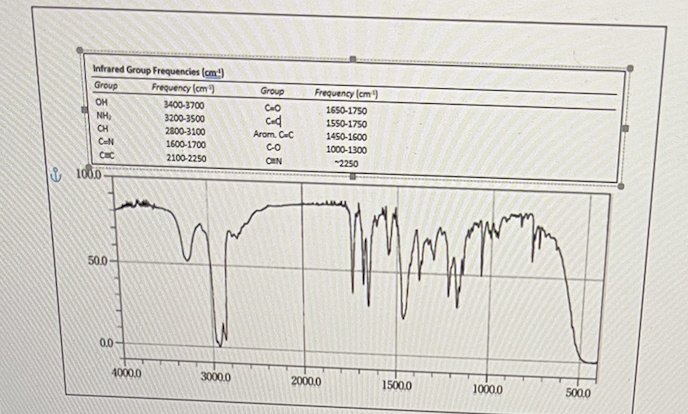
In the infrared spectrum shown below, what represents all of the functional groups observed?
OH, C=O, phenyl, CH
True or False:
Peak asymmetry is calculated as the distance from the center line of the peak to the back slope divided by the distance from the center line of the peak to the front slope, with all measurements made at 1/2 peak height.
false
The peak asymmetry factor, AF, is calculated using the equation
AF = b/a.
Which of the following statements are true?
a. The ideal value is 1, the acceptable range is 0.95 - 1.15
d. A value of < 0.95 indicates fronting, and a value of > 1.15 indicates tailing
e. Column overload can cause fronting while strong interaction with the column packing can cause tailing
Which of the following statements best describes what is involved in creating ions under ESI conditions?
Desolvation, nitrogen gas, high voltage, coulombic explosion
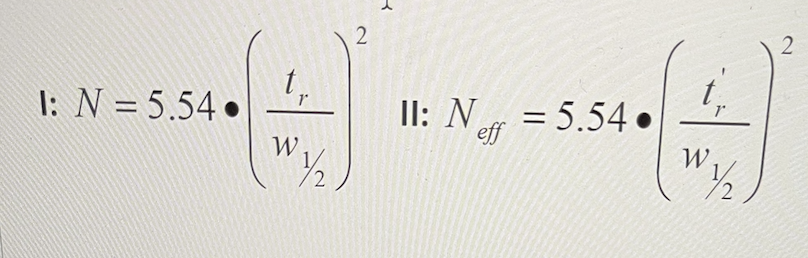
The following equations are used to calculate column efficiency.
What do the terms represent?
W½ = width of the peak at half height, t, = absolute retention time, t' r= relative retention time, Neff = relative efficiency, N = theoretical efficiency
True or False:
Affinity chromatography relies on specific binding by the stationary phase of the target analyte.
True
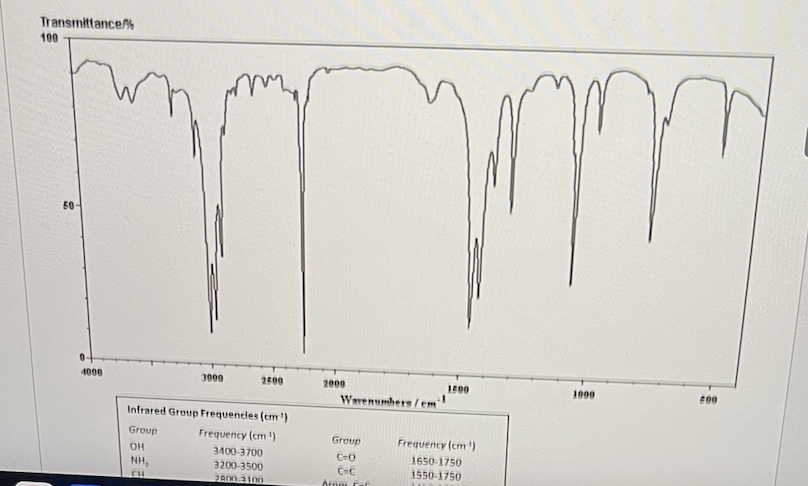
Given the IR spectrum below, what functional groups are present?
CN, CH
True or False:
Fronting of a peak on HPLC can result from column overload
True
Which of the following statements is correct regarding an external standard in HPLC?
A calibration curve is prepared from analysis of different concentrations of analyte
True or False:
Silanol groups do not adversely affect retention of basic compounds in reversed phase HPL, so there is no need for them to be blocked by end-capping with TMS.
False
Pharmacokinetic parameters include, but are not limited to which of the following?
AUC, dose, Cmax, Emax, Cmin, t1/2
Which of the following statements is correct in regard to having an impact on retention and selectivity in reversed phase HPLC?
Hydrophobicity of the stationary phase; pH of the eluant; Temperature; Concentration of organic solvent; column length
A compound has the molecular formula of C27H37CIN204.
How many rings and double bonds does it have?
10
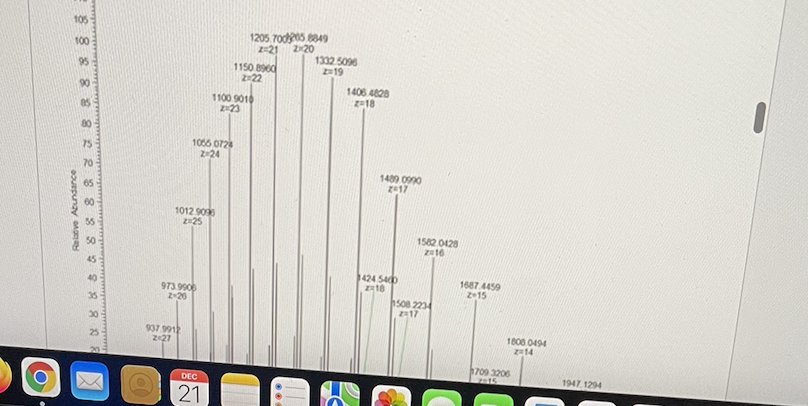
The following high resolution mass spectrum is of a peptide.
What is the mass of the uncharged parent?
Approximately 25297
Rat liver microsomal incubatien of a dopamine agonist with a molecular weight of 654 produces multiple metabolites of m/z 671, 687, 831, and 962.
These represent, in order, which of the following?
addition of one oxygen, addition of two oxygens, glucuronidation, glutathione adduct
The mechanism by which partition chromatography separates compounds is called what?
Dissolution into a liquid phase
Calculate the capacity factor for an analysis where the compound has a retention time of 6 minutes.
An unretained compound elutes at 2 minutes.
K’ = 2
A drug molecule in 20 mL aqueous solution is removed by extraction using 10 mL of an immiscible organic solvent.
How much will be in the organic layer after one extraction, if the partition coefficient is 2?
71%
The nitrogen rule for mass spectrometry tells us which of the following?
An odd nominal mass means an odd number of nitrogen atoms are present while an even nominal mass means there are no nitrogen atoms or an even number of nitrogen atoms present.
The purpose of a guard column in chromatography is to do which of the following?
Protect the main column
True or False:
Increasing column plate height increases the resolution.
False
To remove a metal ion from the aqueous layer, one should do which of the following in the proper order?
Add a chelator, adjust the pH, extract with an organic solvent
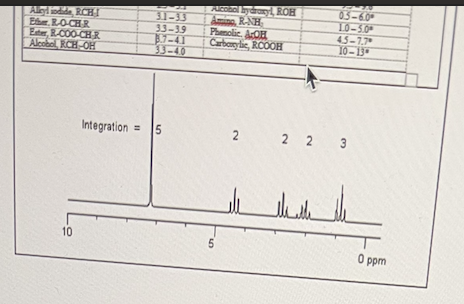
In the following NMR spectra shown below, select the components that the molecule contains.
CH3-CH2, CH2-CH2, C6H5
True or False:
C8, C18 and phenyl are types of chemically bonded phases used in reversed phase chromatography.
True
For normal phase chromatography, which is the order of increasing solvent polarity?
hexane < dichloromethane < isopropanol < methanol
True or False:
Capillary electrophoresis does not need the B term in the Van Deemter equation.
False
Select All That Apply:
A purely silica stationary phase.....
a. is used in normal phase chromatography
b. contains silanols
c. is acidic
d. separates based on absorption
The most sensitive HPLC detectors include which of the following?
Mass spectrometer, radioactivity, fluorescence, electrochemical
True or False:
Increasing the flow rate of the mobile phase in chromatography always improves the separation.
False
True or False:
Open tubular (capillary) GC columns do not need the eddy diffusion term in the Van Deemter equation.
True
True or False:
NMR can be used to aid in the confirmation of a molecular structure.
True
True or False:
TOF mass spectrometry can not produce high resolution mass spectra.
False
True or False:
Gas chromatography will typically use helium, hydrogen, nitrogen, or carbon dioxide as the carrier gas.
False
A basic HPLC consists of which of the following?
solvent delivery system, column, injector, detector, computer controller
A mass spectrum that has a parent ion with an M+2 ion that is about 10% of the parent indicates it has which of the following?
two sulfur atoms
True or False:
Mass spectrometry can be used to suggest a molecular structure.
True
In El mass spectrometry, a molecule is fragmented to produce which of the following?
A neutral radical and an ion
HPLC analysis of the hormone-replacement drug Premarin gives multiple peaks.
Under negative ionization, LCMSMS shows a parent ion for the major component with m/z 347.1.
An M+2 ion is present whose intensity is 7.5% of the parent.
C18H20O5S
In gas chromatography....
Plate height is reduced because A = 0 for open tubular columns
For a reversed phase HPLC....
The lipophilic part of the molecule absorbs onto the lipophilic packing
True or False:
An HPLC radioactivity detector has very high selectivity.
True
True or False:
The McLafferty rearrangement requires an alpha hydrogen.
False
True or False:
A known amount of an internal standard can be added to a blood sample prior to workup to correct for losses during the workup of the sample:
True
Gel electrophoresis is used to do which of the following?
Separate macromolecules Based on both charge and molecular weight
A sample is analyzed on two different columns, column A and column B.
What is the theoretical plate efficiency (number of plates) and which column is more efficient?
Column A: retention time is 3.5 minutes, peak width at half height is 0.1 minutes
height is 0.2 minutes.
Column B: retention time is 3.5 minutes, peak width at half
An unretained sample elutes at 1.5 minutes.
Column A, N = 6787; Column B, N = 1697; column A is the most efficient
Mass spectral analysis of a compound containing two bromines gives a 1:2:1 ion intensity profile because....
There are twice as many molecules that will have both Br-79 and Br-81 as will have two Br-79 and two Br-81 atoms
Calculate the relative retention for two compounds with retention times of 4.5 minutes and 5.0 minutes.
An unretained sample elutes at 1.5 minutes.
a = 1.17