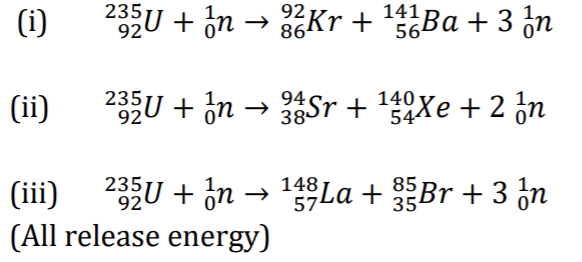Ionising Radiation and Nuclear Reactions
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
Electromagnetic Radiation
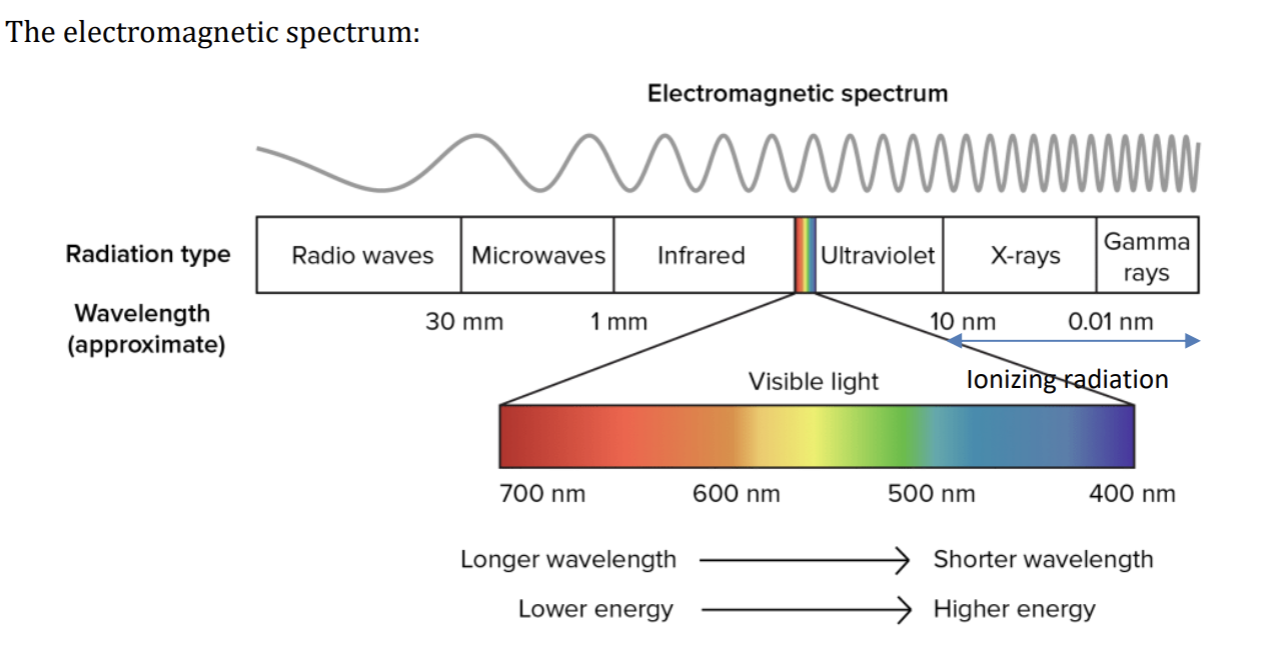
Given off by atoms as electrons jump from one energy level to another, and includes ordinary visible light. Different types of EMR all travel at a speed of 3.00 x 10^8 ms^-1 (in a vacuum) and vary only in their frequency and wavelength.
EMR with high frequency has enough energy to remove electrons from atoms - hence it is called ionising radiation.
The electromagnetic spectrum
Nucear Radiation
Radiation produced as a result of the nucleus of an atom disintegrating is called nuclear radiation - alpha, beta, gamma particles.
Gamma are both electromagnetic radiation and nuclear radiation
Nuclear Radiation and Ionising Radiation
All nuclear radiation is ionising radiation - when it interacts with an atom, it gives outer electrons of that atom enough energy to break way from the atom, thereby leaving as an ion.
Particles knocking off electrons
Both alpha and beta particles knock electrons off their atoms
Alpha → pulls the electrons
Beta → pushes them
Gamma particles give energy direct to the electron by being completely absorbed by it
Alpha and beta particles lose kinetic energy in the process of detaching electrons, slowing down and eventually stopping.
Ionised atoms then have different chemical properties → results in changes to object.
Disintegrating Nuclei and Neutrons
Disintegrating nuclei can also eject neutrons → Zero electrical charge (do not directly cause ionisation in a single step/interaction.
Fast neutrons can be absorbed into a stable atom, making that nucleus unstable and more likely to emit ionising radiation of another type.
Neutrons are penetrating → they have no charge
Atoms
Nucleus → consists of protons (+) and neutrons (0).
Orbiting electrons → have negative charge equal in magnitude to the proton charge.Protons and neutrons collectively called nucleons (mp = mn = 1840 me)
Protons and neutrons are themselves composed of smaller particles called quarks
Number of protons determines the element (atomic number)
Atomic mass number = no. of protons + number of neutrons
isotopes
Strong Nuclear Force
Nucleus is held together by a force stronger than the repulsive force between the protons, and independent of charge (otherwise the neutrons would not be affected)
acts over extremely small distances, so does not affect intermolecular attraction
Stability in nuclei
the closer the ratio of neutrons to protons is to 1, the more stable te nucleus
they tend to redress the balance by ejecting nuclear particles → undergo nuclear decay
Such nuclei is said to be radioactive, ejected particles under go nuclear decay
said to be radioactive + particles ejected are nuclear radiation
Radioactive isotope
A radioisotope
only 300 of the known 1700 known isotopes are stable - the rest are radioactive
every isotope of every element with more than 83 protons is radioactive
Alpha Particles

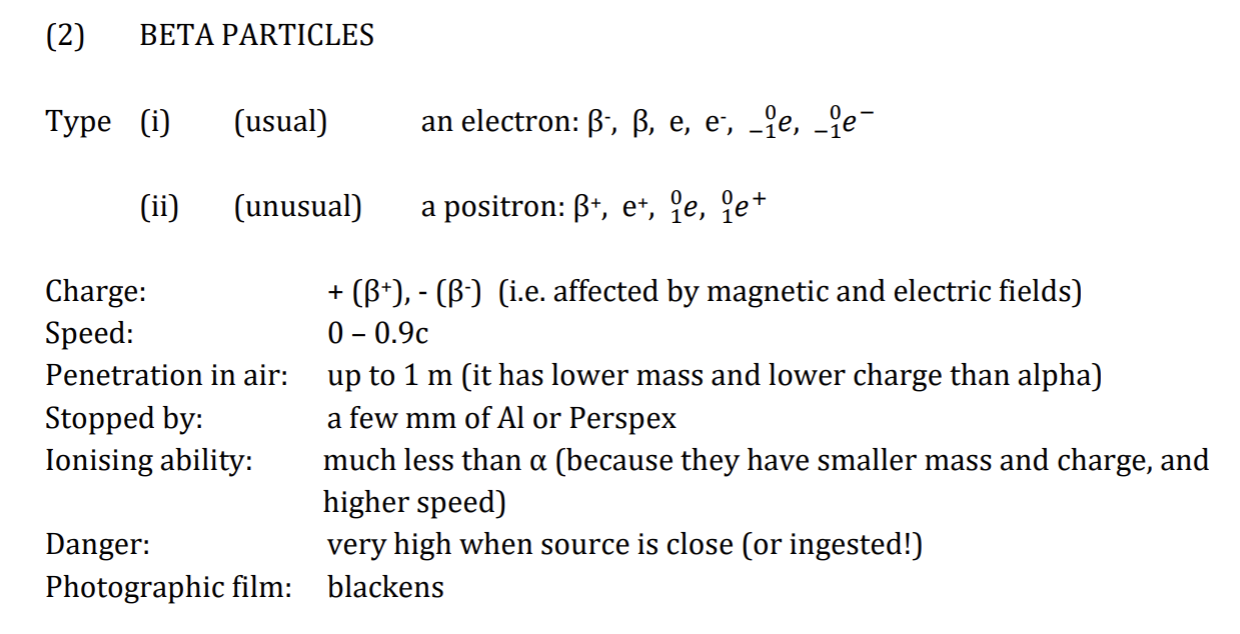
Beta Particles
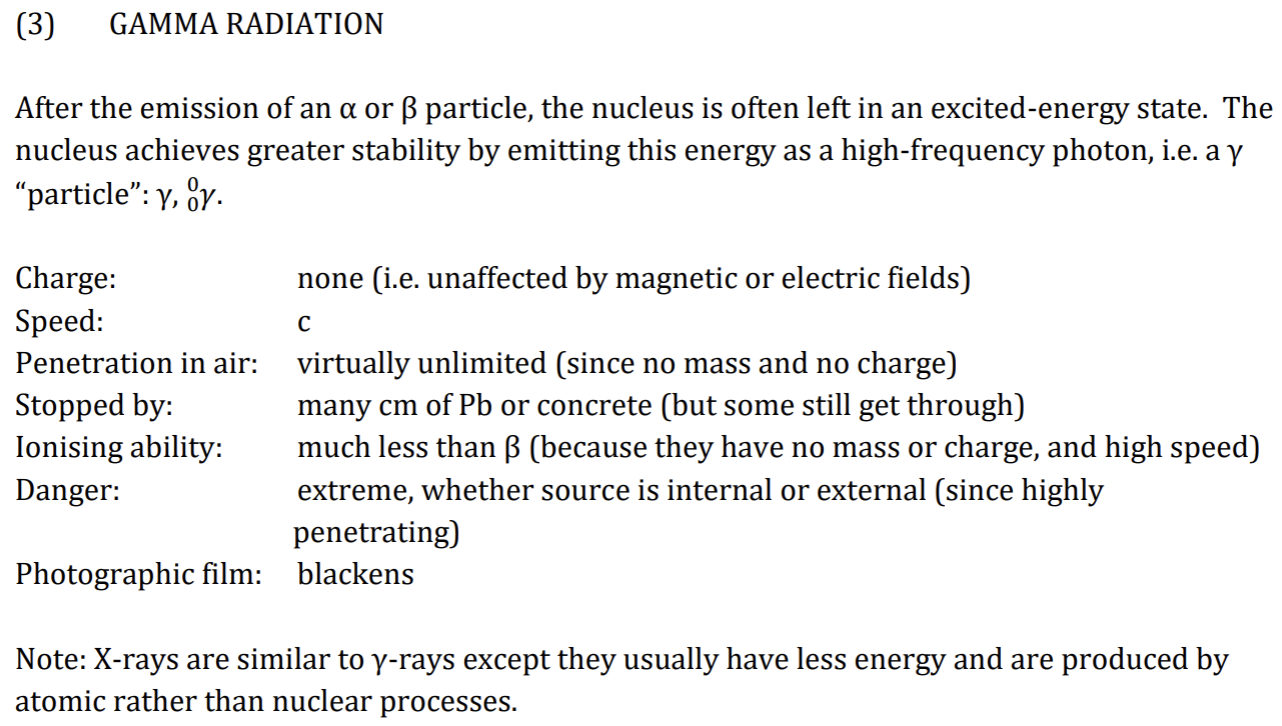
Gamma Radiation
Nuclear Penetrate Diagram
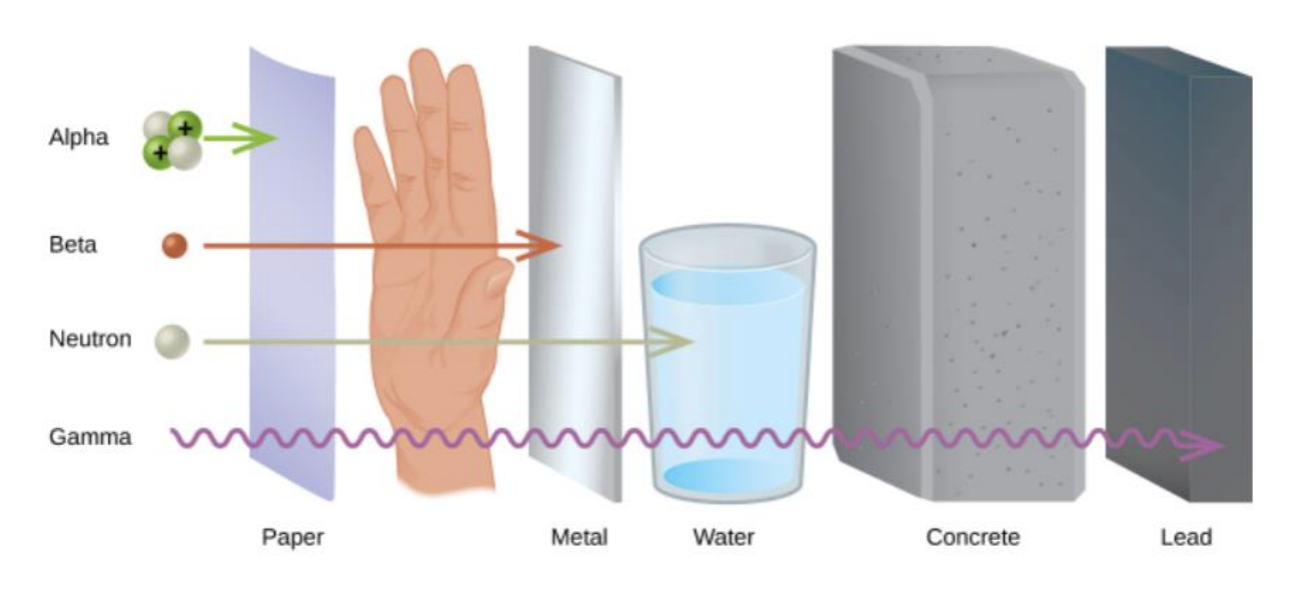
Effects of Radiation on Humans
Ionising radiation causes atoms to lost electrons (become ions) and thus become charged
These ions are chemically reactive and damage or even kill cells, or affect mechanism that regulates cell division - resulting in uncontrolled cell division - a tumour
Ionising radiation can have
somatic effects
genetic effects
beneficial effects
Organs in which many cell divisions occur (e.g. bone marrow, lungs) are more vulnerable to radiation damage
Somatic effects
short term harm caused by damage to or destruction of cells
Genetic effects
Long-term harm caused by damage to cells in reproductive organs (these mutations are then passed on to succeeding generations)
Beneficial effects
e.g. radiation used to kill malignant cancer cells
Time after Radiation
show up about a month after exposure
e.g. damage to reproductive organs, eye lenses, bone marrow, gastrointestinal system, CNS
time between exposure and appearance of damage is called the latent period
Delayed effects = effects that appear months/years after radiations → leukaemia and cancerous tumours
Two main genetic effects of radiation are:
chromosome aberrations (changes in the actual number or structure of the chromosomes)
Genetic mutations that can lead to deformed births in future generations
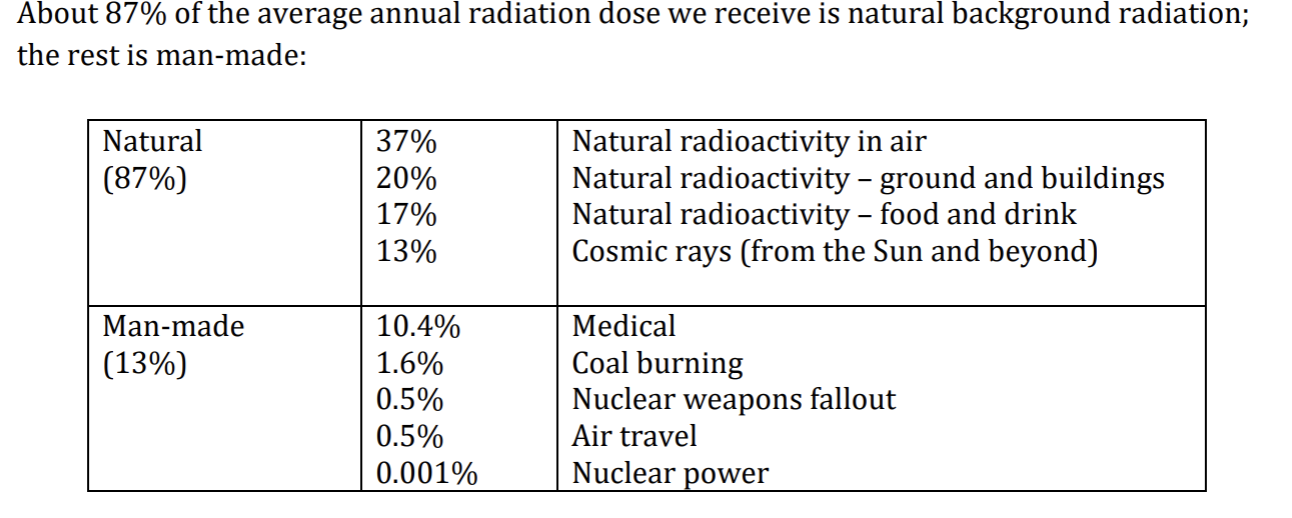
Percent of natural/man made radiation
Beneficial effects of radiation
radioactive tracers e.g. Iodine-123
Smoke detectors
Radiation therapy
PET scans
X-ray pictures
Food irridation
People and radiation
A person does not become radioactive after being irradiated. The radiation will create ions and damage cell biology, but does not cause the production of further radiation.
The only way a person can become radioactive is to ingest a radioactive substance, which emits radiation from within the person until the activity drops to zero.
Nuclear equations
Spontaneous transmutation reactions → Alpha and beta particles
Artificial transmutation → a managed process that changes one nuclide into another (by neutron bombardment)
If nucleus has too many neutrons: reduce the number by changing a neutron into a proton and an electron, + ejecting the electron as a Beta particle.
In most cases, after A or B emission, nucleus is still unstable + loses more energy by immediate emission of Y ray.
Protons + Quarks
Each proton has two up quarks (+2/3) and one down quark (-1/3) →
Charge = positive 1
denoted uud
Neutron and quarks
one up quark and two down quarks
total charge = 0
denoted udd
A neutron can also be viewed as the union of a proton and an electron (or B- particle)
Nucleus + quarks
This explains how an electron can come from the nucleus:
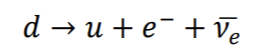
Ve = antineutrino
Similarly a positron (B+) can be emitted from a nucleus
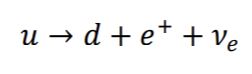
Ve =neutrino
*In nuclear reactions, energy, momentum and charge are conserved. This is achieved by the emission of the neutrino and antineutrino in above reactions.
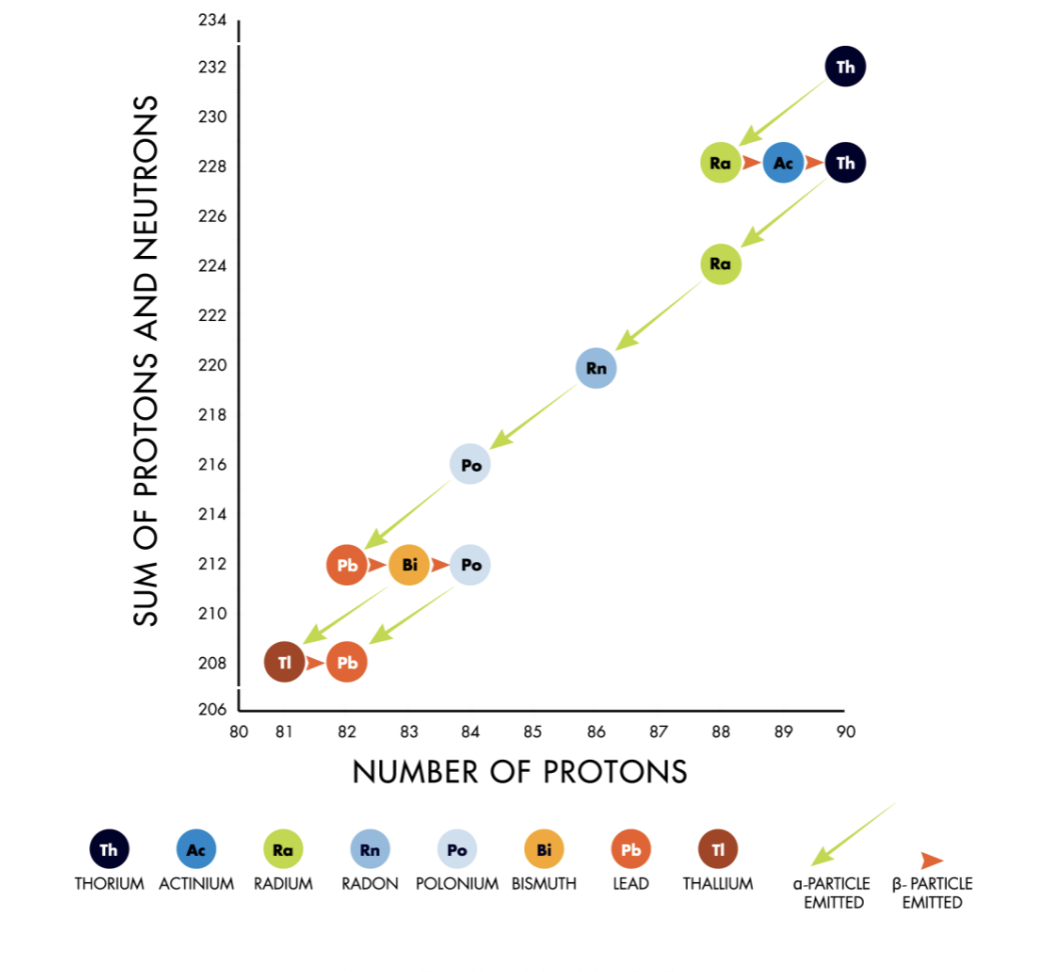
Decay Chains
resulting ‘daughter’ nucleus may be unstable and undergo further decay. This process can continue until stable atom is produced.
Half life of daughter nucleus may be different from mother.
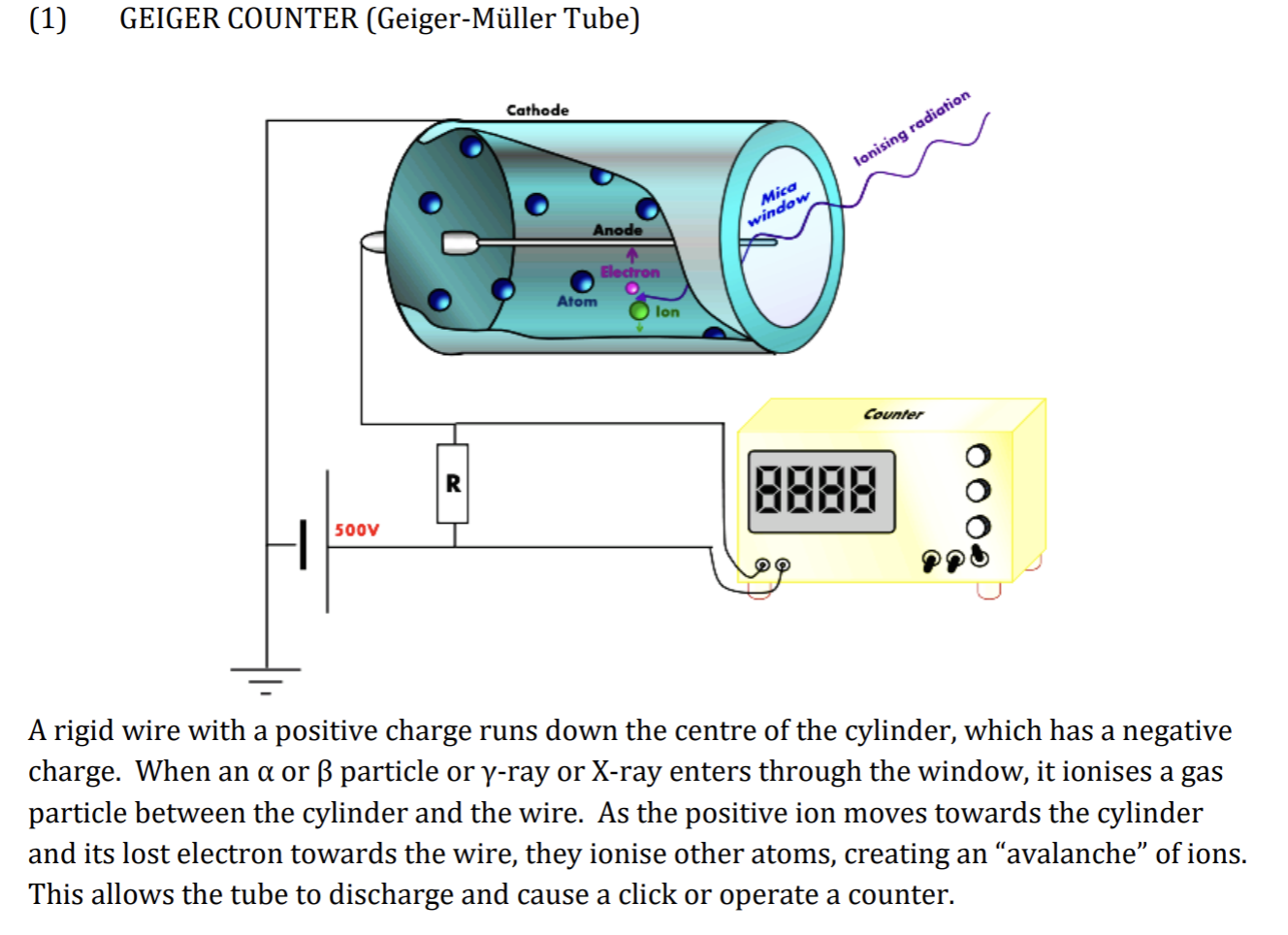
Detecting Radiation
Geiger Counter (Geiger Muller Tube)
Film Badges
Thermoluminescent dosimeter
Wilson Cloud Chamber
Geiger Counter
Film Badges
Principle → Radiation blackens photgraphic film
Filters of various thicknesses are attached to the film badge to distinguish between X-rays and Y-rays and B-rays
Film badges are not accurate
Thermoluminescent Dosimeter
Certain materials store a small amount of the energy absorbed from radioation and release it later when heated in the form of light.
Can detect X, Y and B rays
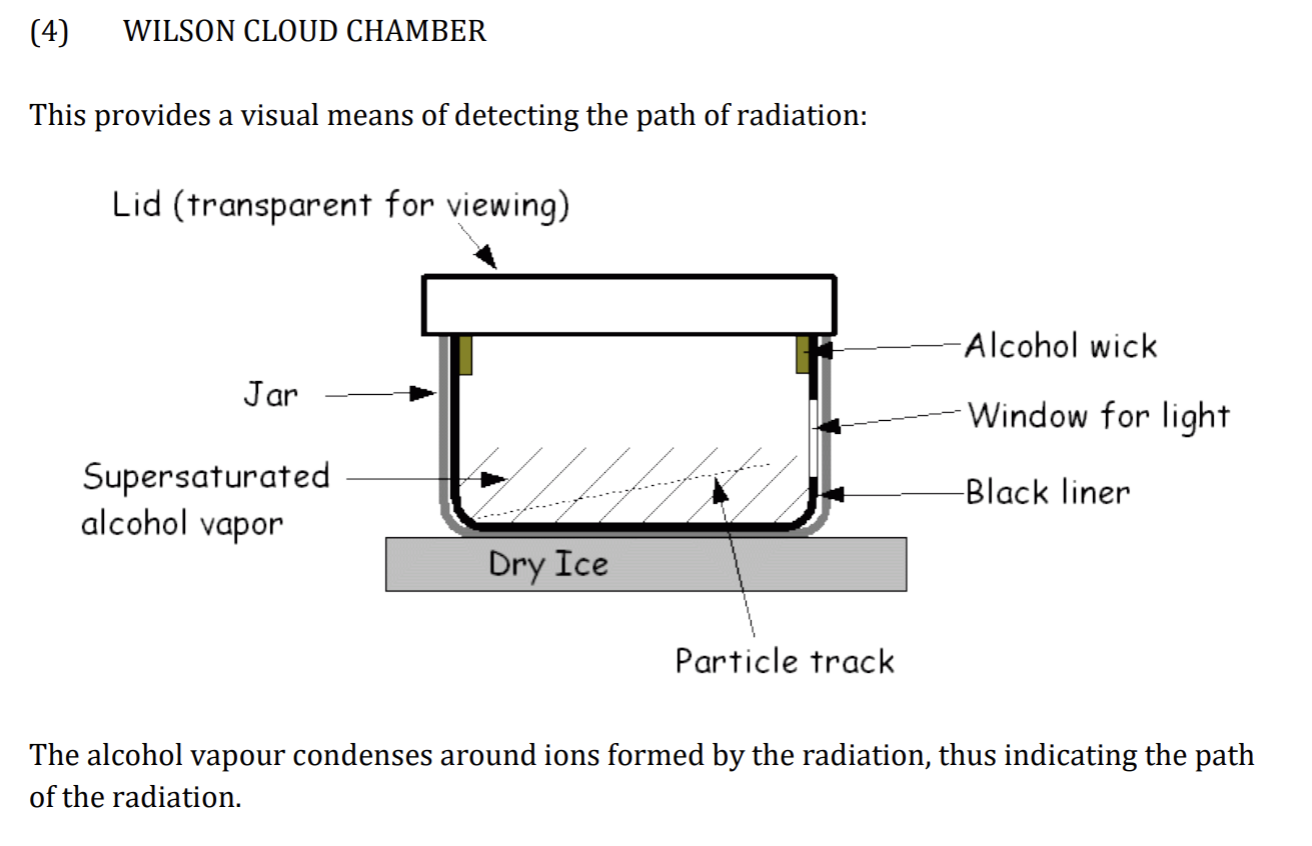
Wilson Cloud Chamber
Radiation Units and Becquerels
Atomic mass unit = 1/12 mass Carbon 12 = 1.6661 × 10^-27
The becquerel (Bq) is a unit of radioactivity
1 Bq = 1 transformation per second
Activity = N/t (number of transformations/time)
The gray
a measure of radioactive absorbed dose
1Gy = 1JKg^-1
Absorbed dose = energy absorbed (E) / Mass of affected body part (m)
Unit = Gy
Sieverts
a measure of dose equivalent
Dose equivalent = absorbed dose x quality factor
1Sv = 1Jkg^-1 x QF
Biological effect of radiation on animal + plant depends on radiation absorbed dose and type of radiation.
Each type has its own quality factor (relative biological effectiveness), which is multiplied by radiation absorbed dose to obtain the dose equivalent.
Lethal Dose
1Sv = massive dose, cause severe illness
>4 Sv = likely to kill
Recommended 1mSv (public) and 50 mSv (radiation ind. workers) / year
LD50 = radioactive dose that gives recipient a 50% chance of survival (around 4Sv)
LD50/25 = 50% will die within 25 days
RADIATION DOSE VARIES INVERSELY WITH THE SQUARE OF THE DISTANCE FROM THE SOURCE:
e.g. if A is x3 as far from source as B, A will recieve 1/9 radiation of B
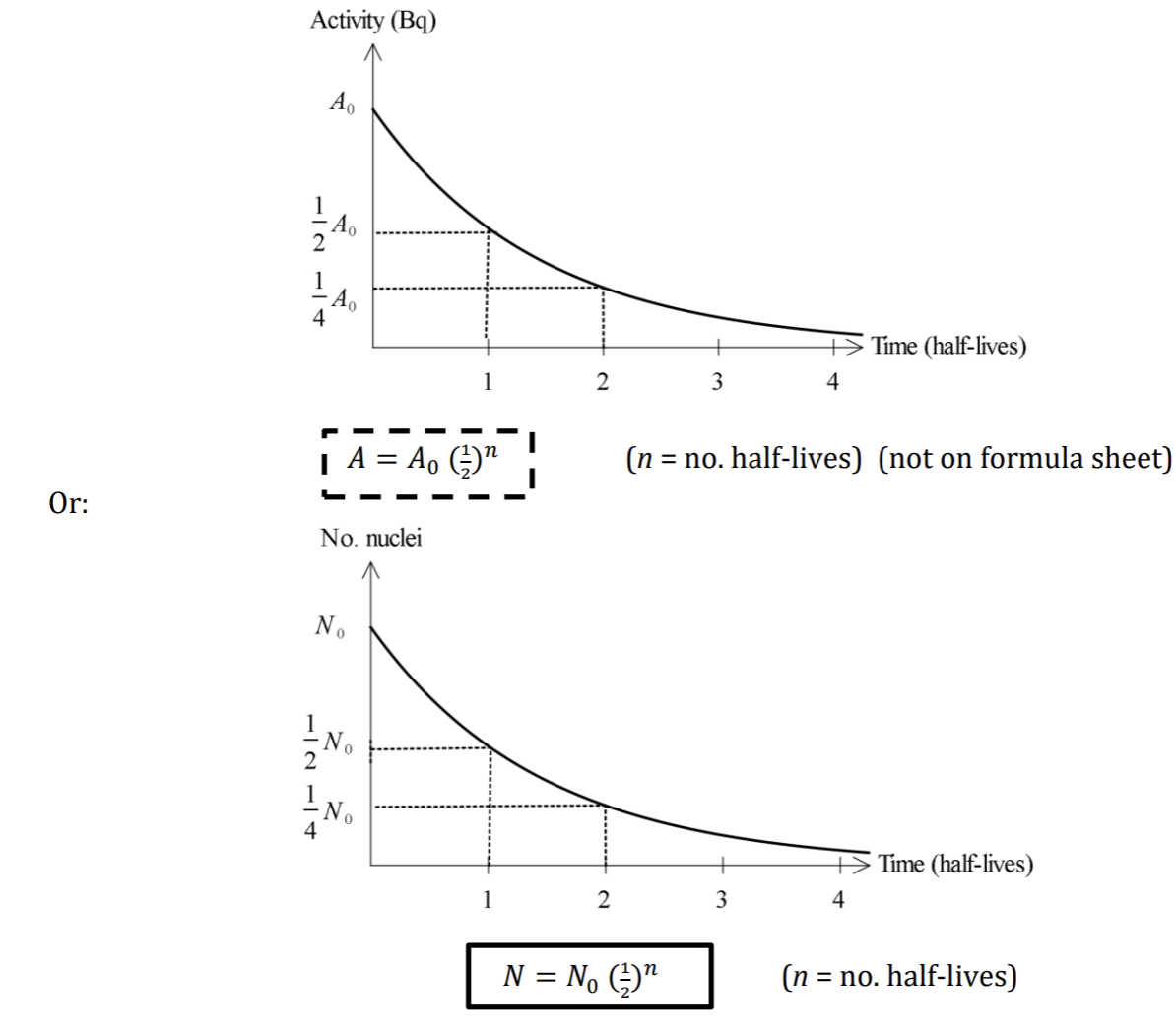
Half-Life
The activity of a sample of radioactive material (i.e. no. of nuclei decaying/second) is proportional to the number of nuclei that are yet to decay
a random process
Half lives vary enormously for different radioactive substances, from microseconds to billions of years. The longer the half-life, the more stable is the isotope.
Half Life Graphs + Formulas
Carbon Dating
The amount of radioactive C14 in atmosphere is constant, the amount of decay being matched by the creation of new C14 by neutron bombardment from cosmic rays.
Formation: N14+neutron → C14 + Hydrogen
Decay: C14 → N14 + B-1
The half life for beta decay is 5760 years
Living things take in C14 and C12 from atmosphere (breathing + eating)
Ceases once they die → amount of C14 declines. By measuring the amount of C14 left in dead wood/skeletons, age can be calculated
**theory assumes proportion of C14 in atmosphere has been constant over the last several thousand years.
Mass Defect
Mass of the assembled particles in the atom is less than the sum of the masses of the individual components
Mass defect = the difference between the isotopic mass of the atom and the sum of the masses of its neutrons, protons and extra-nuclear electrons
Einstein’s equation
E=mc²
Binding Energy
If we regard energy + mass as equivalent: Protons and neutrons lose binding energy as they come together (stone).
Binding energy = the energy that would be required to separate the nucleus into isolated particles.
Particles lose energy when they form a nucleus
Energy must be given to the particles to break up the nucleus
Mass Defect ≡ Binding Energy
Key notes
hydrogen has no binding energy (no mass defect) as its nucleus has only one particle; i.e. mass (H) = mass of one proton
Binding energy per nucleon indicates stability of the nucleus - higher it is = more stable.
electron-volt (eV) is a non-S.I unit of energy 1eV = 1.6022 × 1m10^-19
1u = 931 MeV
Energy released or absorbed in nuclear reactions is often given in MeV
Nuclear Fusion + Fission Overview
B.E nucleon increases as we move along periodic table - up to 26 (iron{ most stable element} ), decreases again after atomic number 26
This is because there are 2 competing forces at work:
protons pushing each other apart
strong nuclear force trying to hold things together
The forces have a crossover point because the strong nuclear force has a very short range but electric force has a long range
as nucleus gets bigger, electric forces starts to win out
Nuclear Fission
Nuclei with atomic number >26 tend to disintegrate to produce more stable nuclei and release energy in the process
The splitting of a nucleus into two lighter nuclei - the sum of the 2 masses of the lighter nuclei is less than that of the original nucleus
Lost mass is released as energy
Nuclear Fusion
Nuclei with atomic number <26 tend to combine to produce more stable nuclei, releasing energy in the process
The union of 2 nuclei into a single nucleus such that the mass of the single nucleus is less than the sum of the masses of the original nuclei
Lost mass = released as energy
Nuclear Fusion and Fission - Energy
Nuclear fusion releases enormous amounts of energy but also requires a large amount of energy → electrostatic repulsion forces between the reacting nuclei must be overcome
More energy is released per nucleon in nuclear fusion than in nuclear fission because a greater percentage of the mass is transformed into energy
Fusion reactions on the sun
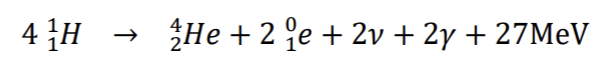
The heat needed to bring the protons close enough to react is available on the sun but not on earth (except by a fission bomb)
Nuclear Fusion Equations
To calculate how much energy is released: calculate the mass difference between products and reactants:
if difference = negative, energy is released in the reaction
if difference = positive, reaction absorbs/requires energy
Chain Reactions
U235, 238, Thorium-232, Plutonium-239 undergo fission when they capture neutrons
e.g: U-235 undergoes fission by capturing slow neutrons: 3 reactions possible:
Average number of neutrons produced in the fission of U235 = 2.5
** The fission products undergo further disintegration, usually B emission
Since each U-235 fission releases more than one neutron, a chain reaction may occur; reaction becomes self sustaining.
Controlled and uncontrolled reactions
Uncontrolled: If released neutrons are continually able to find other nuclei to split, then we have exponential growth; uncontrolled chain reaction, basis of an atomic weapon
Controlled: If chain reaction is strictly controlled so than only one extra neutron is allowed to initiate one further nuclear reaction each time, basis for power station.
Neutron Flux
the number of neutrons emitted per reaction that are suitable for further reaction
Some emitted neutrons may “leak” through the surface, others may be captured by a nucleus but not cause fission.
Critical mass
the mass of uranium beyond which an uncontrolled chain reaction will occur. (an explosion)
If less than the critical mass, most neutrons can escape from the piece of uranium.
If more, most neutrons remain inside the uranium to produce more splittings
Critical mass for U235 = few kilograms, but depends on the size and shape
**presence of other uranium isotopes + impurities affect whether reaction = critical
Nuclear Reactors
the neutron flux is maintained at 1 by varying
ratio of U-235 to U-238
the physical arrangement of the fuel rods
type of moderator used to slow down neutrons (enhancing their chances of being captured by U-235 nuclei)
the first neutron comes from spontaneous fission
a “fast breeder reactor” uses a coolant that isn’t an efficient moderator but uses U-238 as fuel (readily captures high energy neutrons)
Nuclear Reactor Diagram
ion
Neutron Radiation
Neutrons do not cause ionisation themselves, but are likely to collide with hydrogen nuclei in our bodies, releasing high energy protons that becomes dangerous ionising force
LD = 6.5 Sv
Nuclear Tech
Pros
efficient, virtually unlimited supply, “clean”
Cons
dangers of radiation, risk of accidents, problem of disposal of radioactive waste products - depends on radioactive waste and type of radiation it produces
Conventional Tech
Pros
safer, employs more people
Cons
less efficient, chemical pollution, limited fuel supply