VSEPR Theory
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
VSEPR aka…
valence shell electron pair repulsion
What is VSEPR theory?
a model that predicts the 3D shape of a molecule by assuming that electron pairs of a central atom repel each other and arrange themselves to be as far away as possible to minimize repulsion
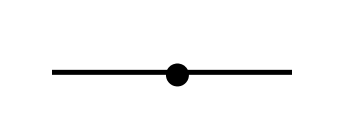
Name the 3D shape
linear
State the # of bonded atoms and # of lone pairs of a linear
# of bonded atoms: 2
# of lone atoms: 0 or 3
State the angle of a linear
180°
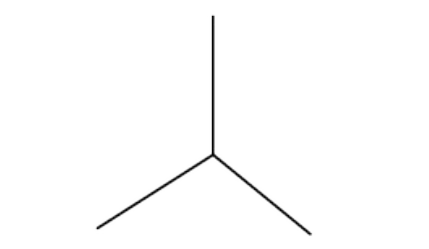
Name the 3D shape
trigonal planar
State the # of bonded atoms and # of lone pairs of a trigonal planar
# of bonded atoms: 3
# of lone pairs: 0
State the angle of a trigonal planar
120°
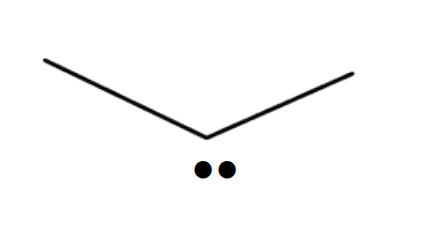
Name the 3D shape
bent
State the # of bonded atoms and # of lone pairs of a bent
# of bonded atoms: 2
# of lone pairs: 1 or 2
State the angle of a bent
<120°
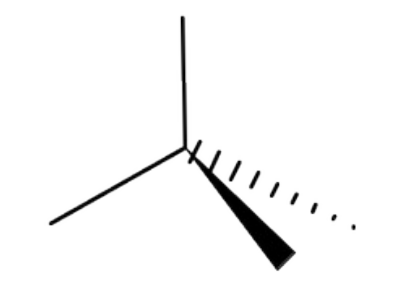
Name the 3D shape
tetrahedral
State the # of bonded atoms and # of lone pairs in a tetrahedral
# of bonded atoms: 4
# of lone pairs: 0
State the angle of a tetrahedral
109.5°
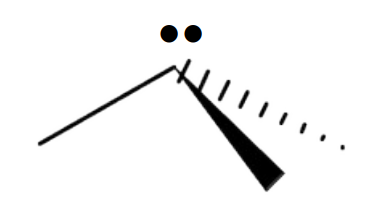
Name the 3D shape
trigonal pyramidal
State the # of bonded atoms and # of lone pairs in a trigonal pyramidal
# of bonded atoms: 3
# of lone pairs: 1
State the angle of a trigonal pyramidal
<109.5°
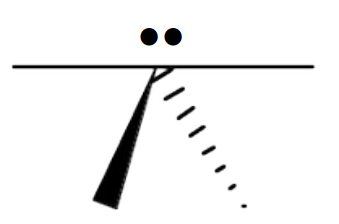
Name the 3D shape
trigonal bipyramidal
State the # of bonded atoms and # of lone pairs of a trigonal bipyramidal
# of bonded atoms: 5
# of lone pairs: 0
State the angle of a trigonal bipyramidal
90°, 120°
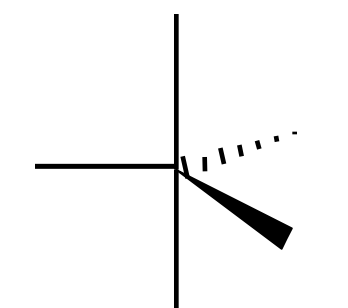
Name the 3D shape
seesaw
State the # of bonded atoms and # of lone pairs of a seesaw
# of bonded atoms: 4
# of lone pairs: 1
State the angle of a seesaw
90°, <120°
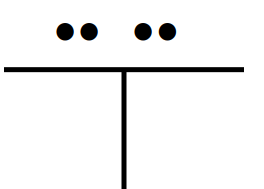
Name the 3D shape
T-shaped
State the # of bonded atoms and # of lone pairs of a t-shaped
# of bonded atoms: 3
# of lone pairs: 2
State the angle of a t-shaped
90°
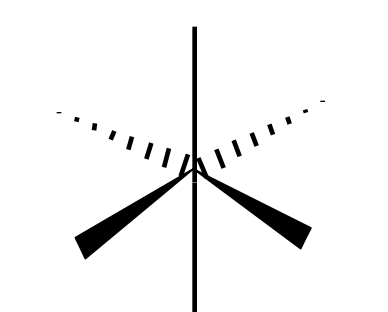
Name the 3D shape
octahedral
State the # of bonded atoms and # of lone pairs in a octohedral
# of bonded atoms: 6
# of lone pairs: 0
State the angle of an octahedral
90°
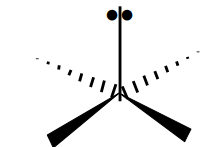
Name the 3D shape (single pair of e- at the bottom)
square-based pyramidal
State the # of bonded atoms and # of lone pairs in a square-based pyramidal
# of bonded atoms: 5
# of lone pairs: 1
State the angle of a square-based pyramidal
90°
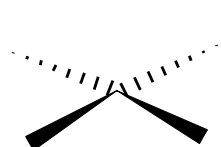
Name the 3D shape (2 lone pairs one at top and the other bottom)
square-based planar
State # of bonded atoms and # of lone pair in a square-based planar
# of bonded atoms: 4
# of lone pair: 2
State the angle of square-based planar
90°