Topic 2: Inorganic Chemistry II - The Origin of Color & Magnetism
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Origin of Color
When an object appears colored, it means that only a portion of the light striking it is absorbed, and the remaining light is reflected
The absorbed light provides energy to excite electrons into higher energy (excited) orbitals
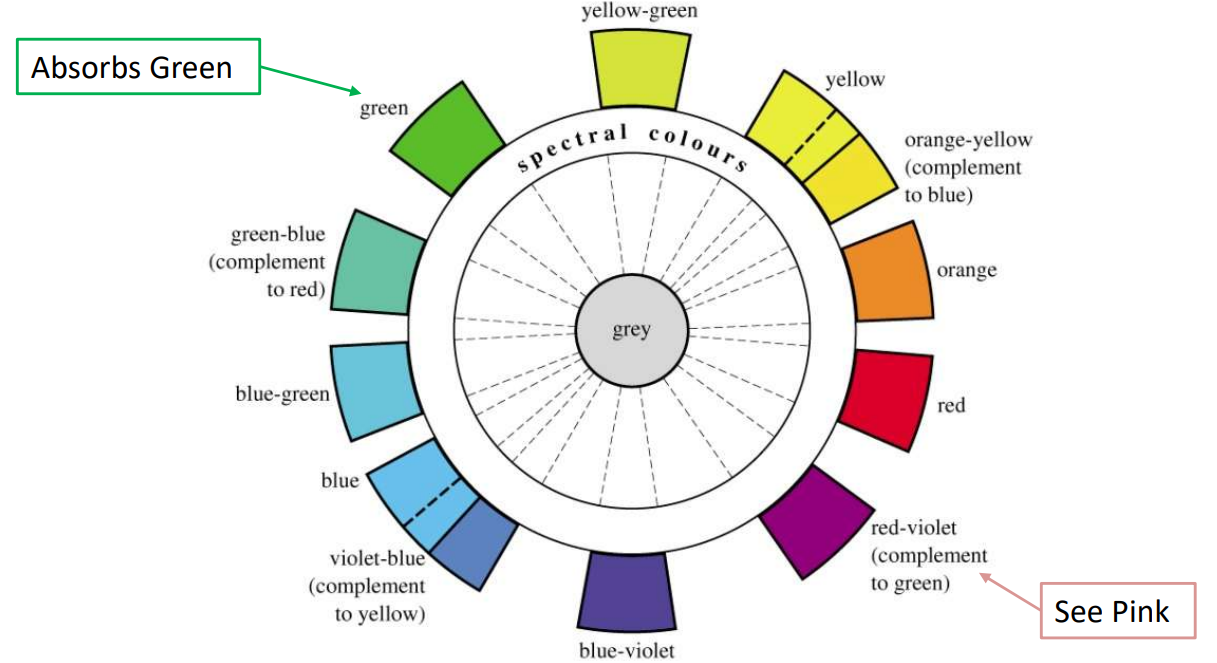
The color perceived is the complementary color to the one absorbed (visualized using a color wheel)
Example: If a compound absorbs green light (around 510 nm), it will appear red-violet or pink
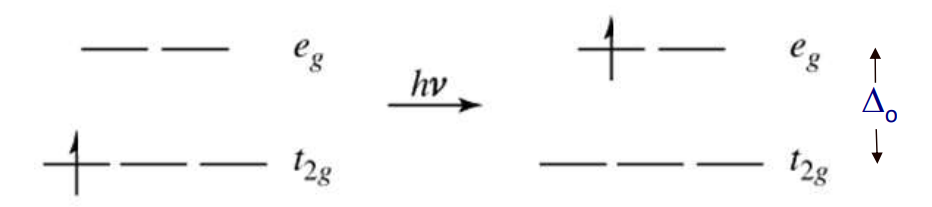
Origin of Color - In Transition Metal Complexes
In transition metal complexes, the color typically arises from electronic transitions between the split d-orbitals, known as d-d transitions
If the energy gap (Δₒ) matches the energy (E) of an incoming photon → the photon is absorbed → an electron in a lower t2g orbital can be excited to the higher eg orbital
Energy-Wavelength Relationship
λ = hc/E
Where:
λ (lambda) → wavelength of light (usually in meters, m)
h → Planck’s constant (6.626 × 10⁻³⁴ J·s)
c → speed of light (3.00 × 10⁸ m/s)
E → energy of one photon (in joules, J)
Δₒ in Transition Metal Complexes
Often falls within the visible spectrum (400–700 nm), causing them to appear colored
Colored Compounds
Transition metals with d¹ through d⁹ configurations usually display d-d transitions (and are colored)
Colorless Compounds
Transition metals with d⁰ or d¹⁰ configurations absorb photons outside the visible spectrum
d⁰ lacks electrons in t2g orbitals
d¹⁰ has t2g and eg orbitals completely filled
Ligand Field Strength Effect on Δₒ
Strong-field ligands → greater Δₒ → higher energy (shorter wavelength) absorbed
Weak-field ligands → smaller Δₒ → lower energy (longer wavelength) absorbed
Spectrochemical Series
CO > CN⁻ > NO₂⁻ > en > NH₃ > H₂O > C₂O₄²⁻ > OH⁻ > F⁻ > Cl⁻ > Br⁻ > I⁻
Exceptions - Charge Transfer and Color
d⁰ or d¹⁰ complexes can be colored due to charge transfer!
Ligand to Metal Charge Transfer (LMCT)
Electron transfers from ligand orbital to metal orbital
Common in high oxidation states (e.g., MnO₄⁻)
Metal to Ligand Charge Transfer (MLCT)
Electron transfers from metal to ligand orbital
Common in low oxidation states (e.g., [Ru(bipy)₃]²⁺)
Beer-Lambert Law
A = log₁₀(I₀/I) = ε·c·L
Molar absorption coefficient (ε), concentration (c), and path length (L)
Calibration Curve
Plotting absorbance vs. concentration gives a straight line to intrapolate the unknown concentration
Diamagnetic
All electrons paired
Weakly repelled by magnetic fields
Paramagnetic
One or more unpaired electrons
Strongly attracted to magnetic fields
Molar Magnetic Susceptibility (χₘ)
Quantitative measure of magnetic property
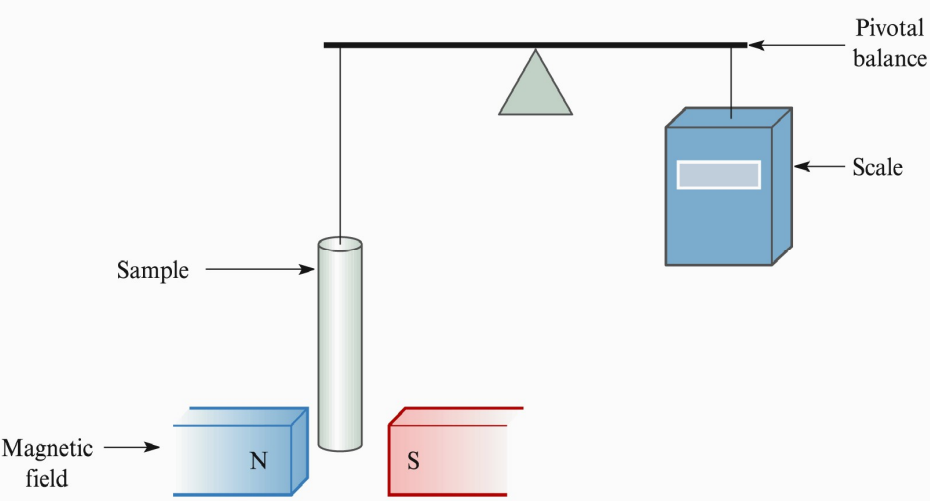
Measurement Method
Suspending a sample of a material between the poles of a magnet
Switching the magnet on attracts a paramagnetic material and weakly repels a diamagnetic material
Causes a change in weight detected by a Guoy balance
Effective Magnetic Moment (μeff) - General Formula
Reported in Bohr magnetons (μB)
Relates to χₘ and T: μeff = 2.828√(χₘ×T)
Where χm = molar magnetic susceptibility, T = temperature
Effective Magnetic Moment (μeff) - Spin-only formula
Reported in Bohr magnetons (μB)
μeff = √(n(n+2))
Where n = number of unpaired electrons
Approximation: μeff ≈ n + 1 (rough estimate)
High-Spin Complexes
Weak-field ligands (small Δₒ), more unpaired electrons, large μeff
Low-Spin Complexes
Strong-field ligands (large Δₒ), fewer unpaired electrons, may be diamagnetic, smaller μeff
Spin-Crossover (SCO)
Compounds that switch between high-spin and low-spin states under heat, light, or pressure
Single Molecule Magnets (SMM)
Retain spin orientation even without magnetic field (e.g., [Mn₁₂O₁₂(Ac)₁₆(H₂O)₄])
![<p>Retain spin orientation even without magnetic field (e.g., [Mn₁₂O₁₂(Ac)₁₆(H₂O)₄])</p>](https://knowt-user-attachments.s3.amazonaws.com/0b130dac-c550-4182-bc10-c61f24cca234.png)