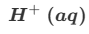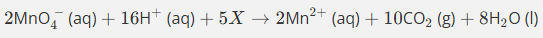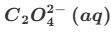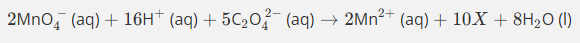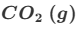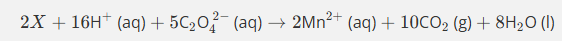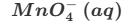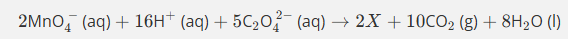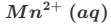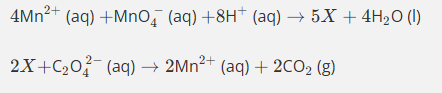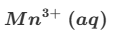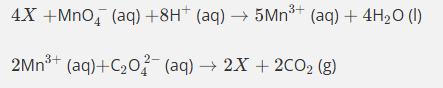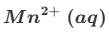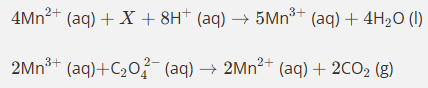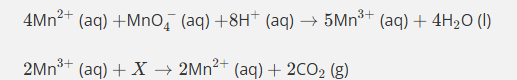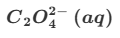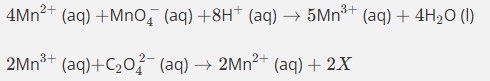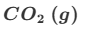Transition Metals as Catalysts
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
51 Terms
A catalyst is…
a substance that increases the rate of a reaction without being used up in the reaction.
BY REDUCING ACTIVATION ENERGY
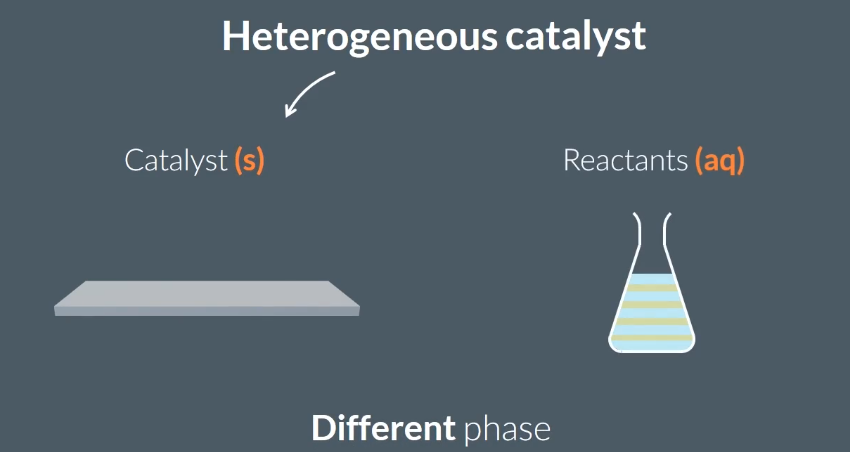
Heterogeneous catalysts are in…
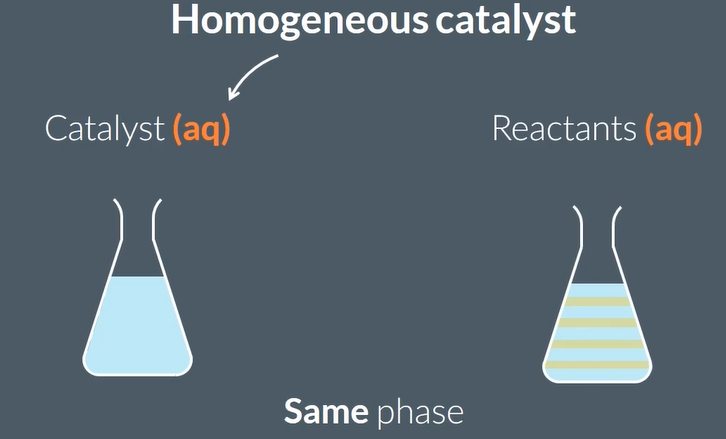
Homogeneous catalysts are in…
a different phase from the reactants.
the same phase as the reactants.
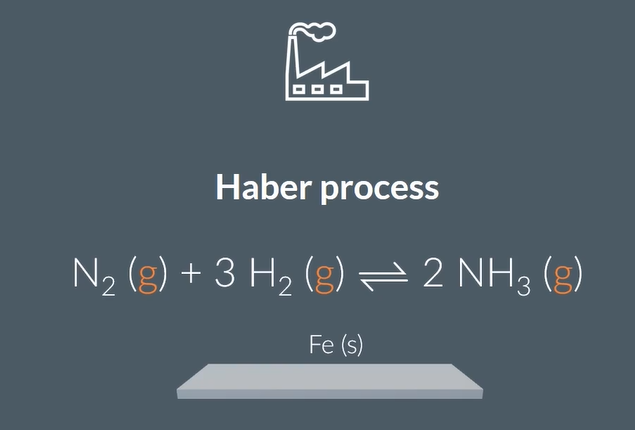
an example of hetrogenous catalyst is in the ….. ……. using a ….. catalyst
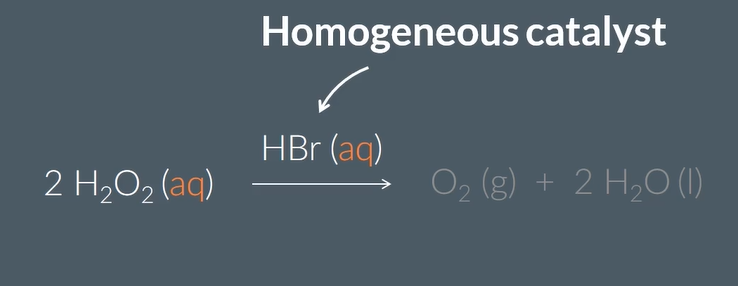
an example of homogenous catalst is in the …… of ……. …… into Oxygen and water using ……. catalyst
Haber process
Fe(s)
decomposiiton
hydrogen peroxide
HBr(aq)
In the presence of an H2SO4 catalyst, alcohols and carboxylic acids react to form esters.
The reactants and the catalyst are both in aqueous solution, so this is an example of...
homogenous catalysis
A catalyst is a substance that increases the…… of a reaction without being used up in the reaction.
Catalysts which are in the same state as the reactants are called……….catalysts.
Catalysts which are in a different state to the reactants are called………catalysts.
rate
homogeneous
heterogeneous
The bit of the catalyst’s surface where the reactants adsorb is called…
active sites
the ….. the surface area of catalyst, the …. number of active sites there can possibly
to maximise this, we apply the catalyst as a …….. coat or a ……
greater
greater
thin
powder
How do Fe catalyst work (not essential)
Solid fe surface provide a suface which the N2 and H2 molecules absorb onto
This breaks their bonds so N atoms can bond to H atoms
Forming ammonia (NH3) molecules at a sugnificantly lower activation energy which ultimatley causes an increase in rate of reaction
Ceramic material is ……. so a hetrogenous catalyst is ……… onto it to maximise the number of …… ……
Ceramic is called a …….. ……….. as its used to hold a catalyst or reactant in place
This is useful to ……… surface area and rate of reaction while also …….. costs instead of expensive metals ie …………..
porous
coated
active sties
support mediums
increase
reducing
platiunum
This sort of catalysis, where the catalyst provides an active site for the reaction, applies to…
heterogeneous transition metal catalysts
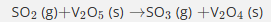
Give the oxidation states of the following elements in the reactants and products.
S: +4 → +6 (oxidised)
V:+5→+4 (reduced)
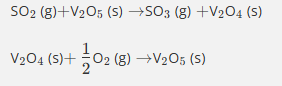
In this reaction, vanadium pentoxide is…
acting as a heterogeneous catalyst.
In the contact process, vanadium pentoxide…
acts as a heterogeneous catalyst.
acts as a homogeneous catalyst.
provides active sites.
uses the variable oxidation states of vanadium to provide an alternative reaction route.
A C D
The contact process allows us to convert SO2 into ………… and ultimately …………. by adding water
This directly is ……… and prodcues hot fog of sulfuric acid.
1) SO3 is dissolved in some …………. to produce H2S2O7
2) then add ……. to the ………… to produce ………….
This allows a safe and esier to contorl reaction
SO3
H2SO4
dangerous
H2SO4
H2S2O7
H2SO4
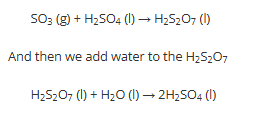
In the exam, you may be asked to remember the equations for the contact process. This quiz will help you to memorise them.
Give the formula and state symbol for compound X in the following equations.
SO2(g)+V2O5(s)→SO3(g)+X
X+12O2(g)→V2O5(s)
V2O4(s)
Give the formula and state symbol for compound X in the following equations.
X+V2O5(s)→SO3(g)+V2O4(s)
V2O4(s)+12O2(g)→V2O5(s)
SO2(g)
Give the formula and state symbol for compound X in the following equations.
SO2(g)+X→SO3(g)+V2O4(s)
V2O4(s)+12O2(g)→X
V2O5(s)
Give the formula and state symbol for compound X in the following equations.
SO2(g)+V2O5(s)→X+V2O4(s)
V2O4(s)+1/2O2(g)→V2O5(s)
SO3(g)
When sulfur in a compound is oxidised in the Contact process (and in general) it is oxidised from oxidation state ……………. to its maximum oxidation state of …………..
+4
+6
heterogenous catalyst provide…… ……. for reactants to ……… onto
fore example in the ……… process, SO2(g) and ½ O2(g) abosrb onto the …………. ……… ctaalyst to form SO3(g)
But industrial measures of SO2 contain impurities eg) AsO2 which ………… the ……….. …………… of catalyst by abosrbing and ……. ……………. making the catalst less effective ……. ……….
This is called P…………..
active sites
absorb
vanadium oxide (V2O5)
block
active sites
not reacting
over time
POISONING
Impurites such as ….. can be removed by spraying ……. over SO2 (g)
dust
water
Explain what is meant by “catalyst poisoning”.
Catalyst poisoning occurs when impurities bind to a catalyst’s active sites and prevent it from functioning.
Negatively-charged particles…
repel
When a catalyst reacts in one step of the reaction, but returns to its original state in a later step, we say that the catalyst is…
regenerated

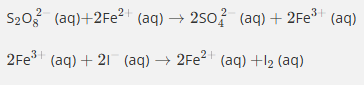
In this reaction, iron acts as a catalyst by…
Select all that apply
attracting the negative reactants.
providing variable oxidation states.
providing active sites.
none of the above.
A
as opposite charges attract lowering activation energy
B
provide an alternte reaction route
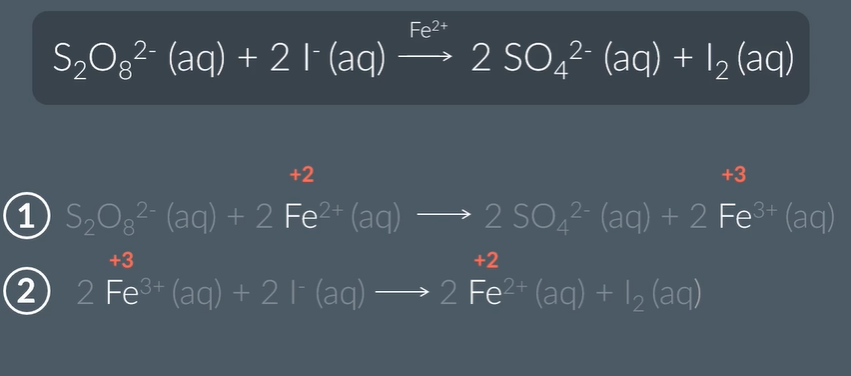
If added Fe 3+ ions instead…
the second step will happen first
Explain how Fe2+ ions catalyse the reaction between S2O82− ions and I− ions.
Fe2+ ions and S2O82− ions attract because they have opposite charges, lowering activation energy
Similarly, Fe3+ ions and I− ions attract because they have opposite charges.
This means that the activation energy is much lower than the activation energy for the reaction between S2O82− ions and I− ions in the absence of a catalyst, because like charges repel.
In addition, iron's variable oxidation states allow it to react with either reactant, and thereby be regenerated.
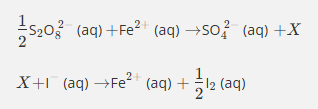
In the exam, you may be asked to remember the equations for the contact process. This quiz will help you to memorise them.
Give the formula and state symbol for compound X in the following equations.
Fe3+
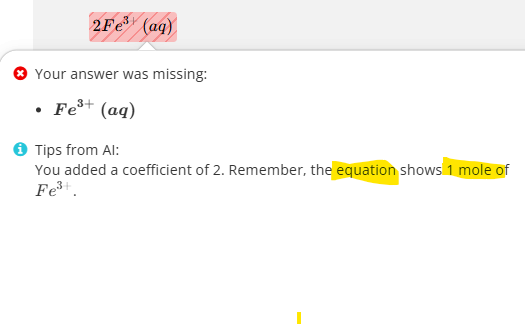
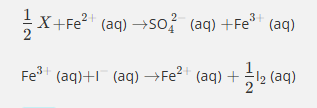
Give the formula and state symbol for compound X in the following equations.
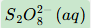
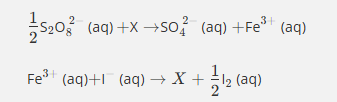
Give the formula and state symbol for compound X in the following equations.
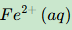
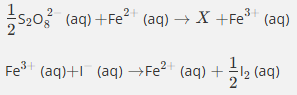
Give the formula and state symbol for compound X in the following equations.
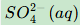
Give the formula and state symbol for compound X in the following equations.
Give the formula and state symbol for compound Y in the above equations.

Give one reason why the reaction between MnO4− and C2O42− is (initially) slow.
The reactants are both negatively-charged. (Therefore, they repel each other.) (This means that the activation energy is high.)
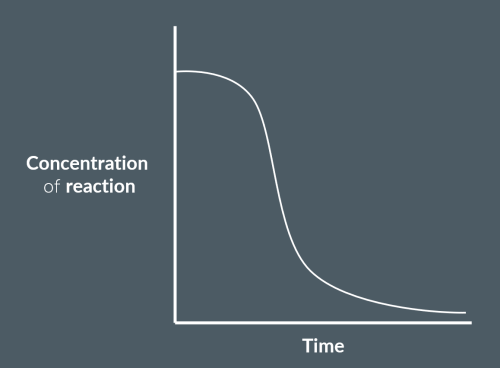
Explain the shape of this graph.
The rate of reaction gradually increases as the catalyst forms.
Then, the catalyst drastically increases the rate of reaction.
Finally, as the reactants are used up, the rate of reaction decreases.
Explain what is meant by the term “autocatalyst”.
A catalyst which is one of the products of the reaction it catalyses.
The reaction between manganate (VII) ions and ethanedioate ions is an example of an autocatalytic reaction. Sketch the graph of concentration of ethanedioate ions over time for this reaction.
S-shaped curve must not rise significantly or fall rapidly initially.
The curve must start on the concentration axis and is initially level but falls sharply after some time before finally levelling off parallel to or on the time axis.
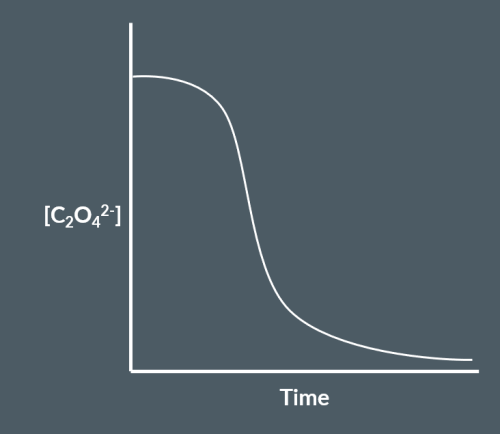
Here’s a sketch of the autocatalytic reaction between manganate (VII) ions and ethanedioate ions. Explain what is happening at each region of the curve.
In region A, the reaction initially proceeds slowly since both reactants are negatively charged and they repel each other. However, after a while, some of the manganate ions will have been converted into manganese ions. Manganese ions act as a catalyst to the reaction that forms them. So, as the concentration of manganese ions increases, the rate of the reaction increases significantly and this is seen in the sharp fall on the curve in region B. Finally, as most or all of the reactants have been used up, the reaction rate decreases to zero and the curve levels off again in region C.
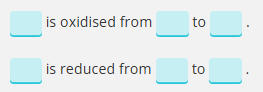
Deduce the oxidation state changes in the following reaction.
MnO4−+C2O42−→Mn2++CO2
eg) C is oxidised and Mn reduced
C; +3→ +4 ( difference of 1)
Mn: +7 → +2 (difference of 5)
therefore C needs to react 5 times as much as Mn
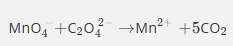
This equation is only partially balanced. Finish balancing it using the lowest whole number coefficients.
Remember that this equation is taking place in acidified aqueous solution.
Do not give state symbols
The first equation produces 5Mn3+ but the second equation requires only 2Mn3+.
So we multiply the first equation by 2 and the second equation by 5 so that the Mn3+ cancels.
This gives us 8Mn2++2MnO4−+16H++C2O42−→2Mn2++10CO2+8H2O
And this is the same as the original equation which produced the catalyst. Remember, the catalyst provides an alternative route for the same reaction.
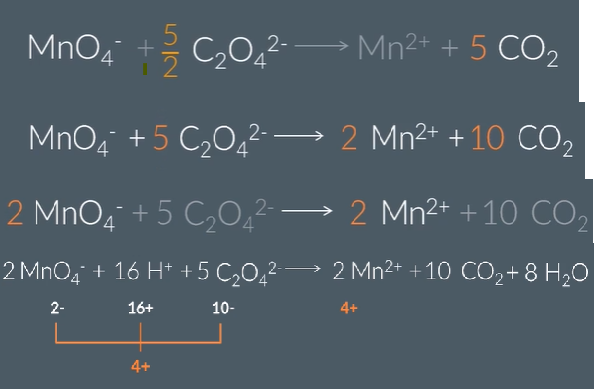

Use the oxidation state method to balance this equation.
(1rst step Mn becoming a catalyst)
Do not give state symbols
Mn2+ oxidised to Mn3+ in products (difference of 1)
Mn in MnO4- has oxidation state of 7+ so is reduced to 3+ in Mn3+ producst (difference of 4)
Thefroe need 4Mn2+ and 5Mn3+ altogther in producs as there are 5 moles in reactants altogether
Then balance )2 by adding h20 by adding on products then add H+ ions onto reactants
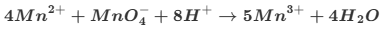
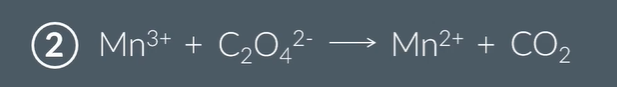
How do you balance this equation then?
Mn3+ is being reduced to Mn2+ so is oxidised witha difference of 1
C being oxidised from +3 to +4 in CO2 products with a difference of 1
as you need ½ C2O4)2-, you times by 2 tow get the 2Mn3+ 2Mn2+ 2CO2
Givin an already balanced equation of 6+ on 2Mn3+ and 2- of C2O4 2- altogether +4 on reactants and +4 on productes (2 Mn2+)
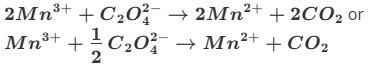
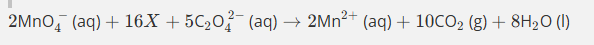
Give the formula and state symbol for compound X in the following equation.
2MnO4−(aq)+16X+5C2O42−(aq)→2Mn2+(aq)+10CO2(g)+8H2O (l)