Biochem Rutgers Murphy Final
1/265
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
266 Terms
conversion of acetyl-CoA to malonyl-CoA is inhibited by
Glucagon, Epinephrine, Palmitoyl-CoA
The recycling rate of triacylglycerols to fatty acids is inhibited by
insulin
Lipolysis is used to
degrade triacylglycerols
Glycerol from the hydrolysis of triacylglycerols is transported by the blood to the
liver
What is the major mechanistic difference in purine and pyrimidine biosynthesis
purines are synthesized on the ring of ribose
How many FADH2 molecules would be produced in the oxidation of palmitic acid (16:0 carbons)
7
Myelin sheath
surrounds nerve cell axons and facilitates nerve impulse transmission
patients with familial hypercholesterolmia
- have missing or defective LDL recpetors
- are homozygous or heterozygous for a nonfunctional LDL receptor gene
- Have very high levels of serum cholesterol
______ are the principal transporters of cholesteryl esters to tissues
low density lipoprotein
the benzene ring of the aromatic amino acids is formed by
shikimate pathway
the pathway in which lipoproteins are transported from the liver to cells is referred to
endogenous pathway
in simple diffusion a solute
is propelled by random molecular motion
inorganic nitrogen is initially assimilated into which of the following amino acids
glutamine
which of the following is not one of the ketone bodies
b-Methylglutarate
all of the following are purine bases except
thymine
prostaglandins are involved in
ovulation, inflammation, and digestion
the principal means of producing glycerol in the body
glyceroneogenesis
depending on an animal's metabolic needs, fatty acids may be converted to triacylglycerols, energy or -------
used in membrane synthesis
Lipid molecules
fatty acids, steroids, isoprenoids, waxes
neutral fats belong to ------
triacylglycerols
------- is used to transport fatty acids into the mitochondria
carnitine
SAM is a methyl donor in the synthesis of -----
methionine, creatine, phosphatidylcholine
fatty acid synthesis begins with the carboxylation of acetyl CoA to form ------
Malonyl-CoA
Membrane remodeling
requires phospholipases and acyl transferases
------, a product of the oxidation of odd-chain fatty acids, is converted to succinyl-CoA
propionyl-CoA
saturated fatty acids containing up to 16 carbon atoms are assembled in
cytoplasm
which of the following is an alpha-keto acid/alpha-amino acid pair used in transamination
oxaloacetate/aspartate
Function of lipids
energy storage, components of biological membranes, insulation, source of acetyl-CoA
Why do most naturally occurring fatty acids have cis double bonds in them
the double bond results in a bend in the molecule lowering the melting point of the molecule.
The fixation of nitrogen requires
Fe-Mo protein, Fe protein, ferredoxin
In plants fixed nitrogen is assimilated into
Glutamine
The major route for protein degradation is
ubiquitin proteasomal system
Fatty acid long carbon tails are referred to as
Acyl
The double bonds in naturally occurring fatty acids are usually _______ isomers.
Cis
Protein channel requiring transport mechanisms include
Primary active transport and facilitated diffusion
All of the following statements concerning folic acid are true EXCEPT:
Folic acid requires intrinsic factor for its absorption in the intestine
the triacylglycerol cycle is
- a mechanism that regulates the level of fatty acids that are available to the body for energy generation
- a mechanism that hydrolyzes triacylglycerol
- converts fatty acids to triacylglycerols
waxes
are esters formed from long-chain fatty acids and long-chain alcohols
who makes who? estradiol vs. testosterone
the enzyme aromatase converts testosterone to estrogen
the function of dolichols is to
transport sugars in glycoprotein synthesis
how many isoprene units does a diterpene contain
4
glycolipids differ from sphingolipids in that they contain no
phosphate
Intermediates in the nonoxidative phase of the pentose phosphate pathway include all of the following except?
dihydroxyacetone phosphate
which of the following is an oxidizing agent in fermentation
pyruvate
gluconeogenesis occurs primarily in the
liver
in nucleosome structure, the histone ----- aids in stabilizing the wrapping of DNA around the protein octomer
H1
Which of the following does not contribute to the noncovalent interactions that stabilize the helical strands of DNA
phosphodiester bonds
which of the following describes the flow of genetic information as stated in the central dogma of molecular biology
DNA--> RNA --> Protein
Nucleosomes are composed of all of the following except
H5
Which of the following is NOT a second messenger molecule/system?
Steroids
which of the following statements is NOT true of mRNA
prokaryotic mRNAs are capped with 7-methylguanosine
the DNA "backbone" is composed of
a phosphodiester bond between 3' and 5' hydroxyl groups of adjacent deoxyribose residues
which of the following amino acids is capable of forming a N-linked glycoprotein
N
which of the following amino acids is capable of forming an O-linked glycoprotein
T
what creates the difference between the blood-group antigens, A, B, and O
all types are O-linked, but possess different sugars
An important role of glycosylation of proteins in the body is
create a diversity of structural possibilities and facilitate extracellular recognition
glucose-6-phosphate is a substrate in which of the following processes
gluconeogenesis and glycolysis
the bifunctional enzyme, PFK-2 would have which of its domains active in response to insulin
the kinase domain
all of the following are important components of the electron transport chain except
Coenzyme A
Evidence supporting the chemiosmotic theory includes all of the following except
the pH of a weakly buffered suspension of mitochondria rises when O2 added
Rap video
energy production
what effect would inhibition of complex 1 of the electron transport chain have on the regulation of pyruvate dehydrogenase (PDH) complex
the increase in NADH would decrease PDH activity
the allosteric regulation of which of the following enzymes is important in the regulation of glycolysis
hexokinase, PFK-1, pyruvate kinase
which of the following enzymes will catalyze a committed step in glycolysis
phosphofructokinase-1
which of the following would be a signal that would lead to an unphosphorylated form of PFK-2/FBPase-2 enzyme
insulin
w-6 fatty acids
have a double bond six carbon atoms from the methyl end of the chain
which of the following is not likely to be a naturally occuring fatty acid
C14H29COOH
A membrane's fluidity is largely determined by the percentage of
unsaturated fatty acids
the role of very low density lipoproteins is
transporting of lipids from liver to tissues
the transamination of alpha ketoglutarate leads to the production of
glutamate
amino acids whose degradation yields a-ketoglutarate include all of the following except
leucine
which of the following amino acids cannot be used to provide an intermediate of the citric acid cycle
Lysine
all amino acids can be synthesized from intermediates of
the glycolytic pathway, the pentose phosphate pathway and citric acid cycle
Which of the following compounds in NOT a precursor for nucleotide biosynthesis
fumarate
The first step of nitrogen fixation is the
Transfer of e- to ferredoxin from NADPH
_________ ATP molecules are required to convert one mole of nitrogen to two ammonia molecules.
16
Nitrogen fixation requires Mo and _________________ as cofactors
Fe
The glutamate family of amino acids includes all of the following except________________
Lysine
Asparagine is formed from aspartic acid and _______________
Glutamine
All of the following are directly involved in the synthesis of deoxyribonucleic acid except_________
HGPRT
Besides PRPP, how many molecules are involved in forming a pyrimidine nitrogenous base?
2
Which of the following statements about hormones are correct?
1. Hormones can be peptides, steroids, or amino acid derivatives
2. Hormones can stimulate the synthesis of target proteins through the activation of specific genes.
4. Hormones can increase the cellular uptake of metabolites
Under which conditions are the lac structural genes expressed least efficiently?
No cAMP, no lactose
In addition to the insulin receptor being able to signal to mTOR and upregulate things like lipid biosynthesis, ribosome biogenesis and translation, another biosenser in the cell can sense energy levels and nutrient availability. This biosensor's name is...?
AMPK
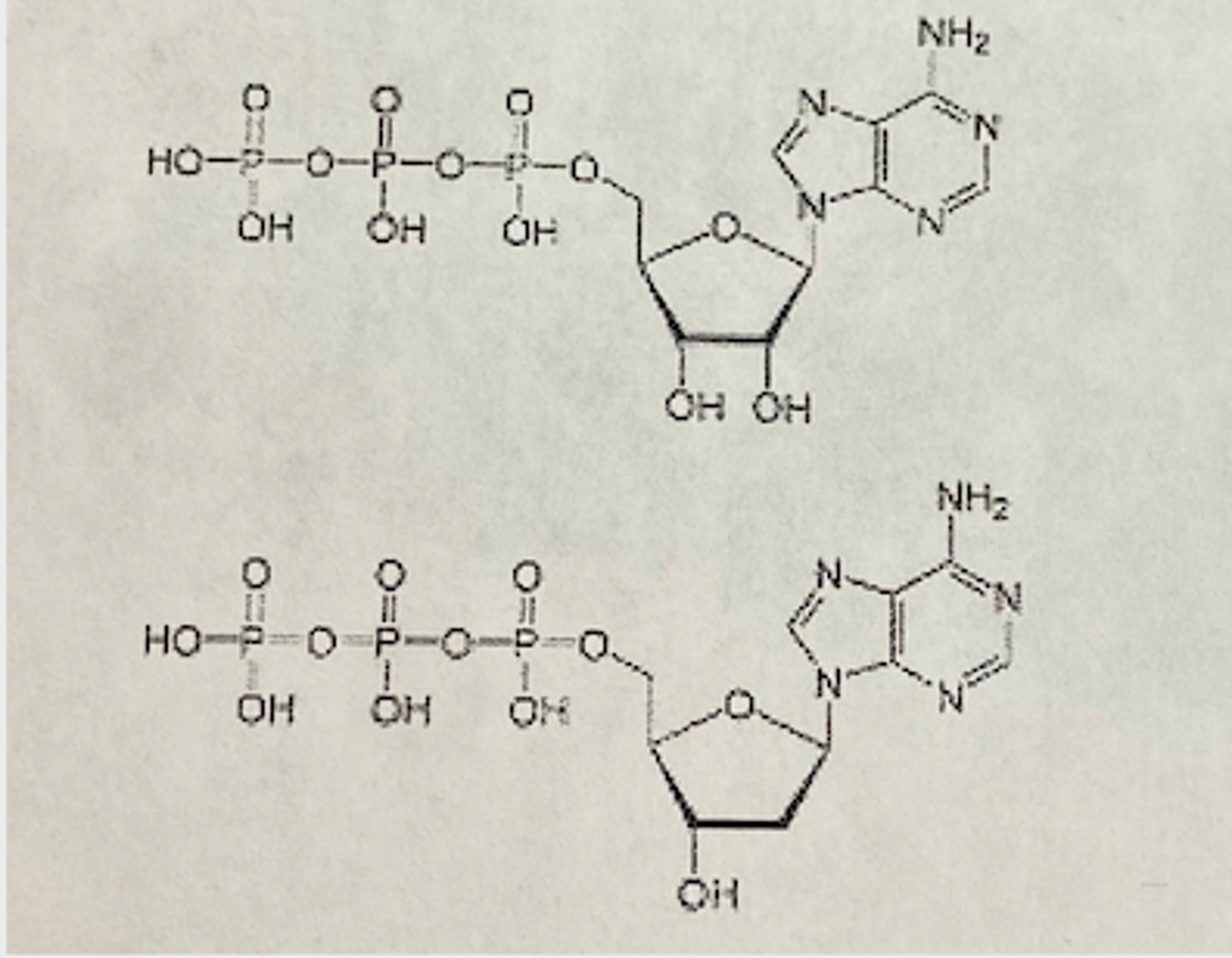
In the image below which of the following represents nucleotide triphosphate that would be incorporated into mRNA?
The upper diagram
Posttranslational modifications include all except
Removal of introns
The process of translation is extremely accurate (1 mistake in 10,000) due to
codon/anti-codon interactions and amino-acyl tRNA synthetases
Which of the following best describes the role of EF-Tu (elongation factor Tu) in elongation of protein synthesis?
It is involved in the correct binding of amino acyl tRNA to the A site of the ribosome
Which of the following statements about the ubiquitination of proteins are true?
1. Ubiquitin is a relatively small protein that is widely expressed in eukaryotic cells
2. It is covalently attached to target proteins in an ATP-dependent rxn
4. Target selection is determined by ubiquitin-protein ligases
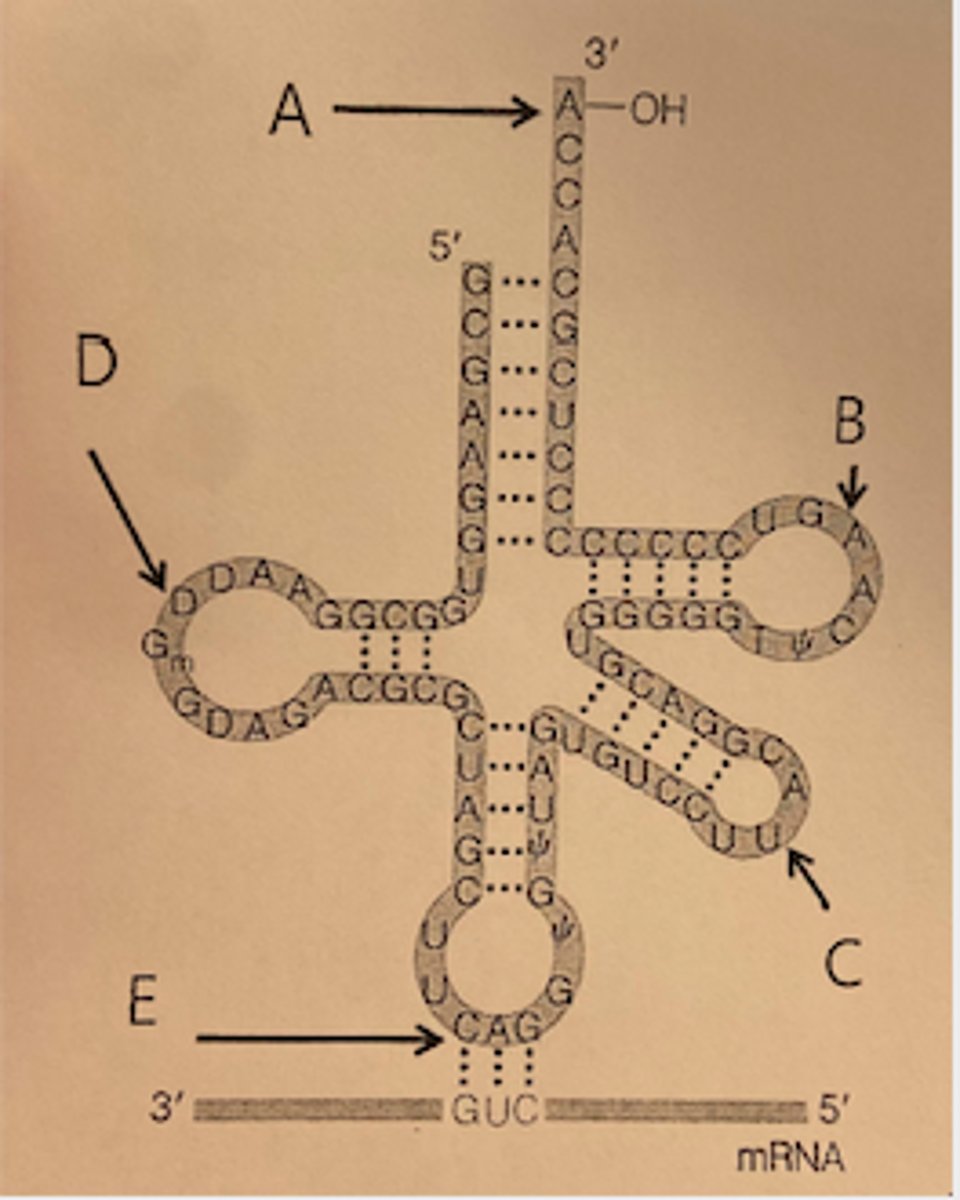
Which of the following letters in the diagram below is pointing to the loop that forms the anticodon on tRNA?
Letter E
A researcher will do which fo the following to elucidate how a known steroid receptor protein sends its signal through the cell ultimately changing transcription of a gene. Pick the best choice
Perform a western blot comparing phosphorylated and unphosphorylated versions of a potential kinase target of the receptor molecule
The classic experiment that demonstrated that radioactivity labeled viral DNA transforms bacterial cells was performed by
Hershey and Chase
Why would siRNA be preferred over a chemical inhibitor that targets the same molecule?
Off targets of chemical inhibition may occur where siRNA is specific to a desired mRNA
A western blot is used to detect level of which of the following?
Proteins
Determine the primary sequence of a protein that would be produced from the following non-coding DNA sequence: 5' - GGCCATTTCCCGTAT - 3'
Ile-Arg-Glu-Met-Ala
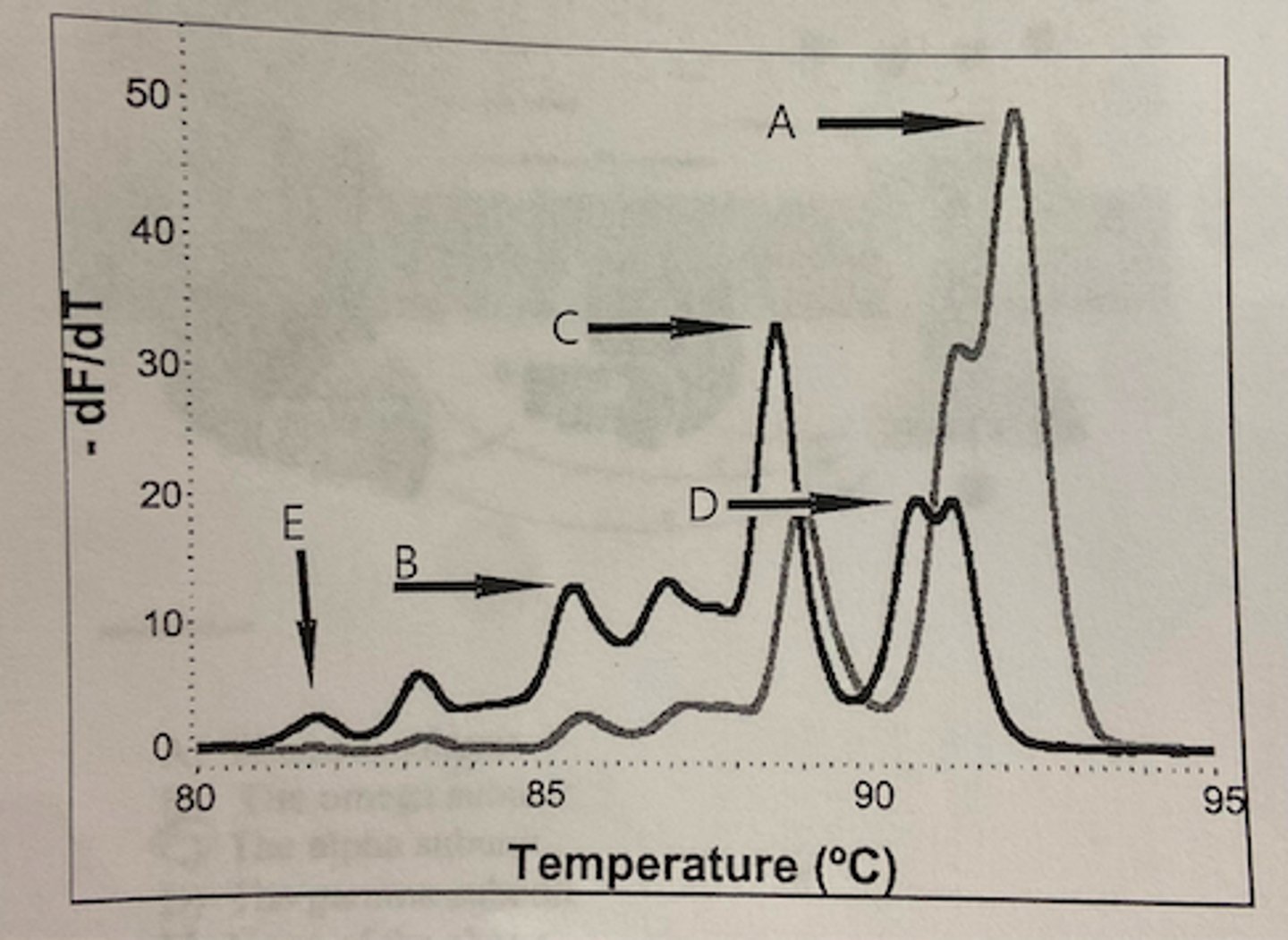
If the length of the resulting real-time PCR products are identical use the melt curve data below. If peak A consists of DNA with only G-C base pairs and peak E consists of DNA with all T-A base pairs, which of the remaining peaks has the next greatest amount of A-T verses G-C base pairs?
The peak labeled B has the next highest amount of A-T compared to G-C base pairs
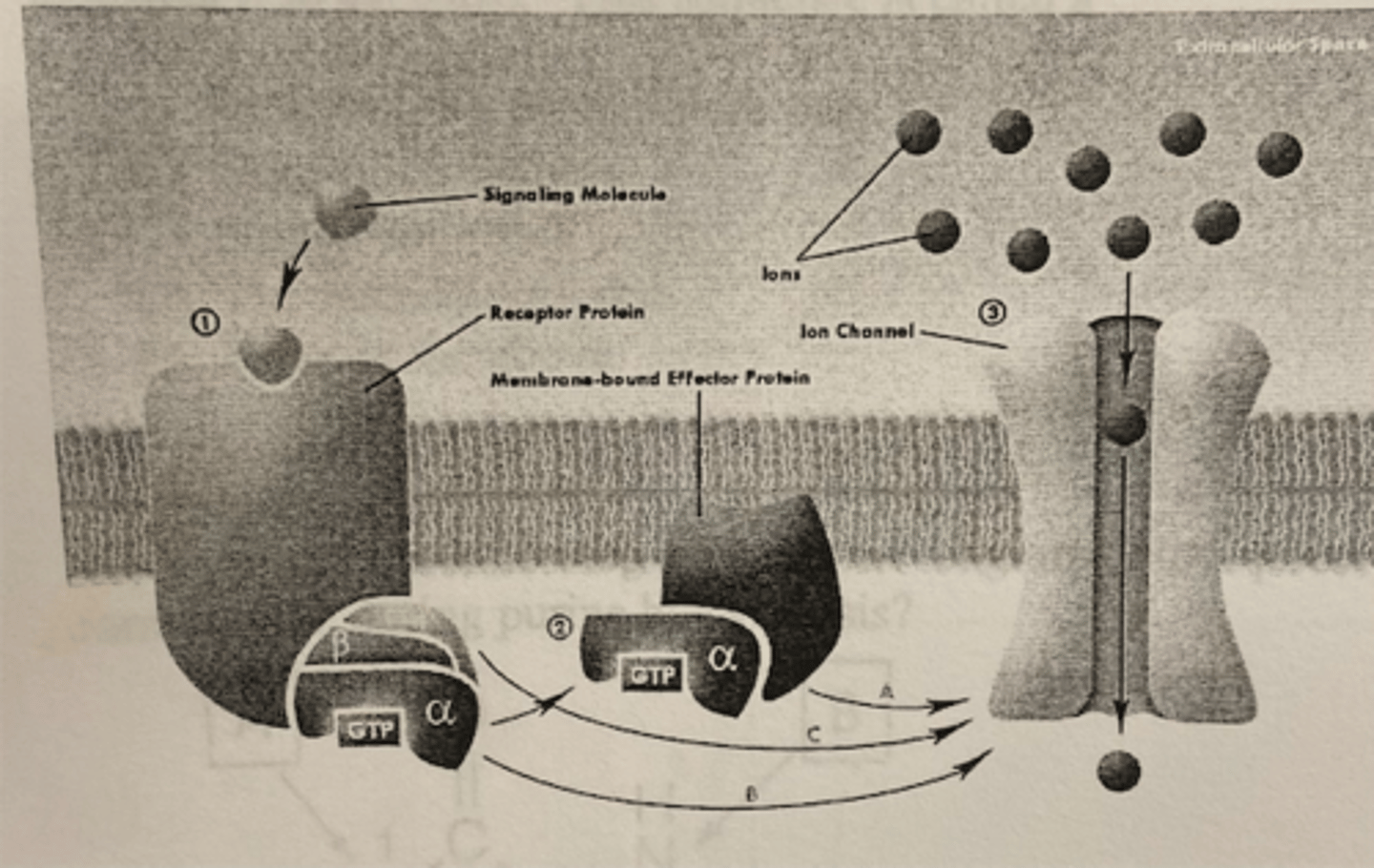
Which of the trimeric subunits will hydrolyze GTP to GDP?
The alpha subunit
The following is a hypothetical experimental result obtained after treatment of a cell type with a toxin (Tox) compared to control (control). A western blot was run and the results of the western blot are shown below. Which of the following experiment(s) is/are necessary to run to definitively show that the increase of protein was due to mRNA stabilization only?
- Run real-time PCR on the mRNA of the gene and show no change in level
- Perform a luciferase assay on the promoter of the gene and show no change in level

A protein destine for insertion into the rough endoplasmic reticulum contains a sequence that instructs it to go there. This sequence is called a
Signal Sequence
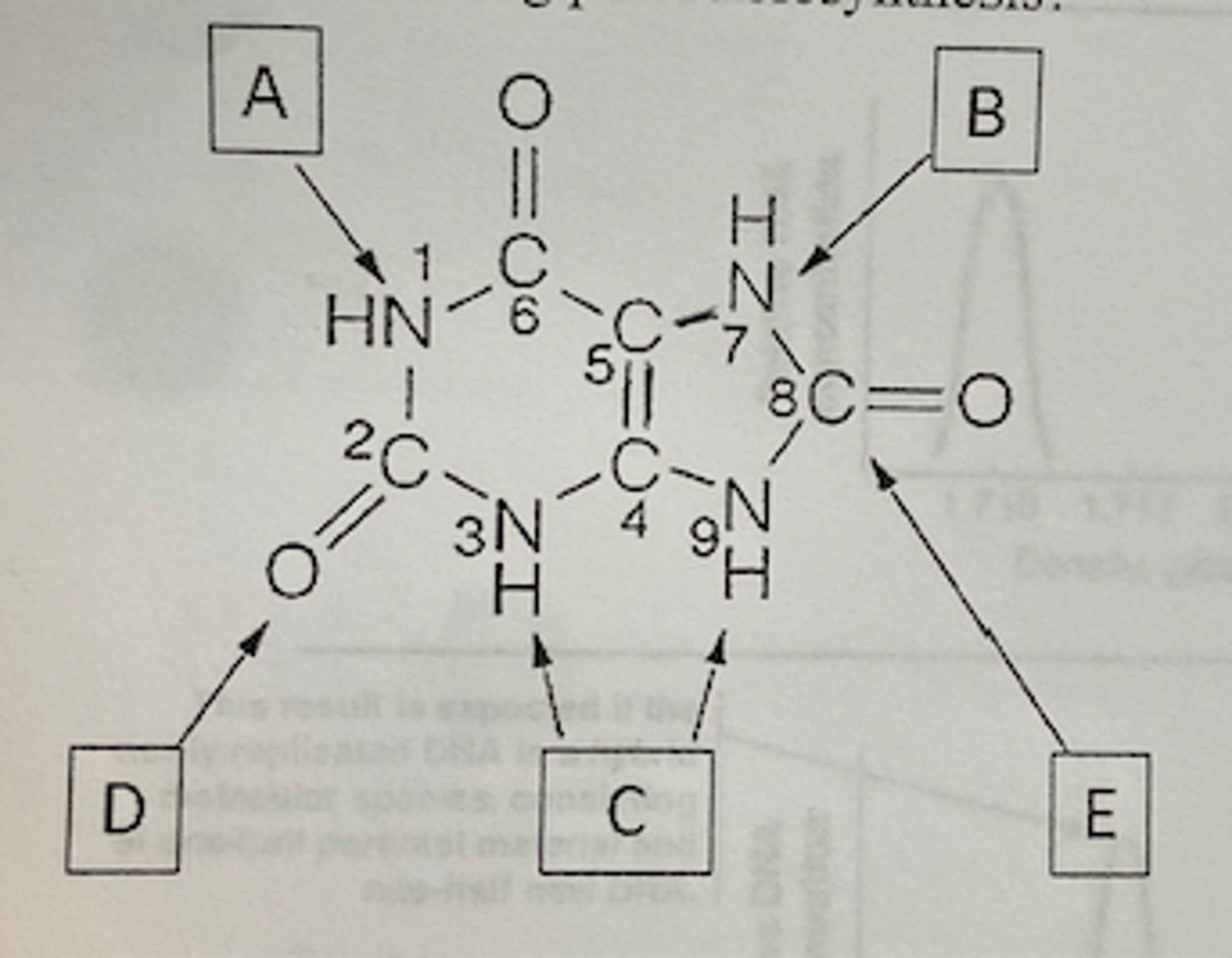
Which of the following letters in the diagram below point to a carbon donated by 10-formyl-THF during purine biosynthesis?
Both D and E