Introduction to Biological Molecules: Study Guide (Unit 1)
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
What are the four organic building blocks & what larger molecules do they each make?
Sugars/Carbohydrates [makes polysaccharides, glycogen, and starch (in plants)]
Lipids/Fatty Acids (makes fats and membrane lipids)
Amino Acids (makes proteins)
Nucleotides (makes nucleic acids)
Generic formula for carbohydrates
Formula: C(H2O)n , where
n = the number of carbon atoms (can range from 3-12 carbons)
Most common groups based on the number of carbons
3 carbons: triose (glyceraldehyde)
5 carbons: pentose (ribose)
6 carbons: hexose (glucose)
Hydroxyl group
-OH

Why are hydroxyl groups important for sugars?
Each one on a sugar is potentially a site where a chemical reaction can occur
What is a monosaccharide and what are it’s two major uses?
A single carbohydrate molecule
Two major uses of monosaccharides:
Making ATP via cellular respiration
Building larger polysaccharides
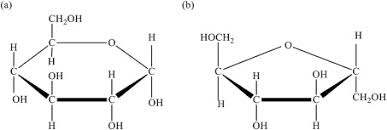
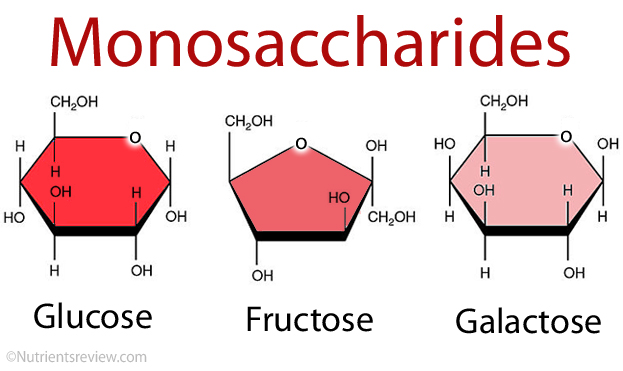
What are the three major monosaccharides in the body?
Glucose
Fructose
Galactose
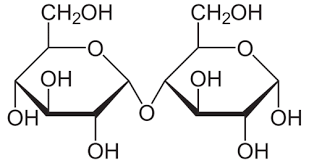
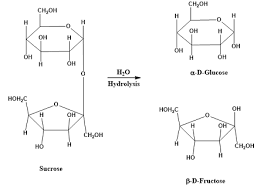
Disaccharide
Two monosaccharides (sugars) linked together by a glycosidic bond.
What are the three major Disaccharides in the body?
Sucrose; table sugar (glucose + fructose)
Lactose; milk sugar (glucose + galactose)
Maltose; malt sugar (glucose + glucose)
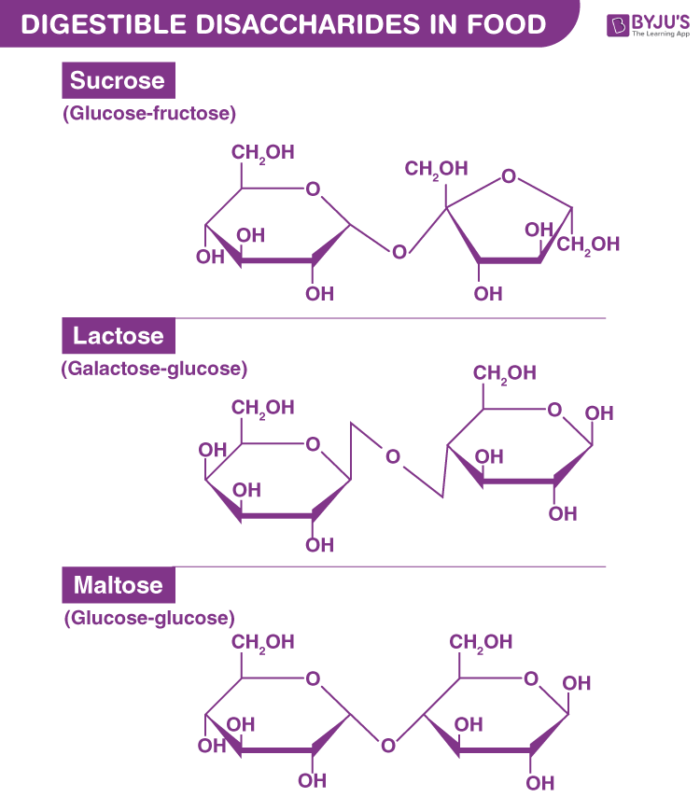
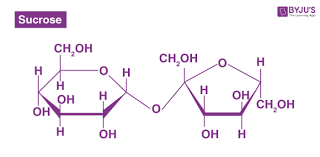
What two monosaccaride sugars create the disaccharide sucrose (table sugar)?
Glucose + Fructose
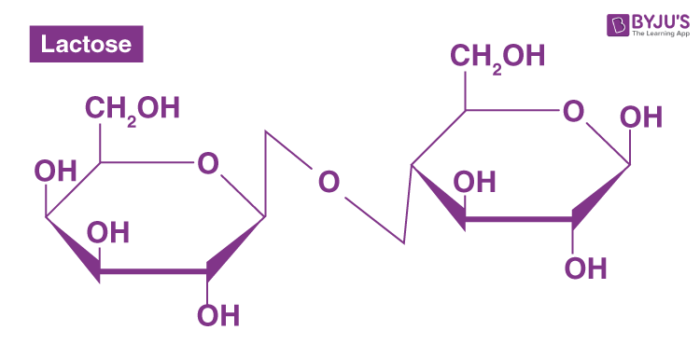
What two monosaccaride sugars create the disaccharide lactose (milk sugar)?
Glucose + Galactose
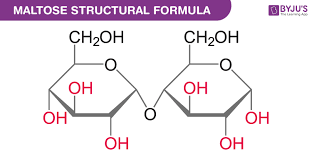
What two monosaccaride sugars create the disaccharide maltose (malt sugar)?
Glucose + Glucose
Oligosaccharide
A sugar chain containing between 3-10 sugars
Polysaccharide
A sugar chain containing more than 10 sugars
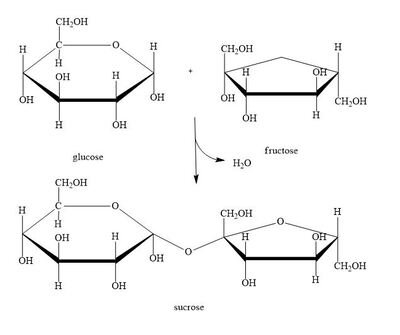
Condensation/Dehydration synthesis reaction
A glycosidic bond is formed between two molecules (monosaccharides) by removing a water molecule
Hydrolysis reaction
Broken bonds from condensation being reformed by adding water back
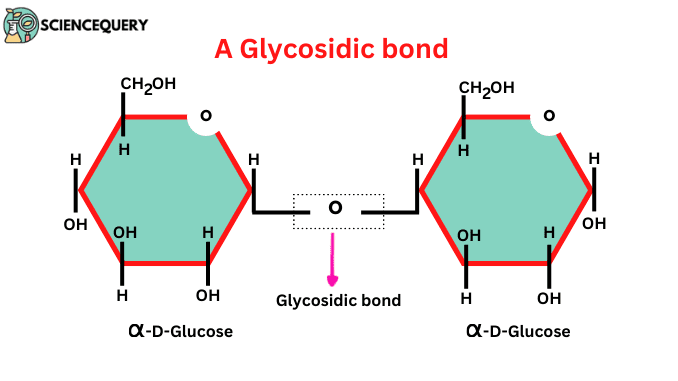
Glycosidic bonds
Bonds formed by condensation between two monosaccharides
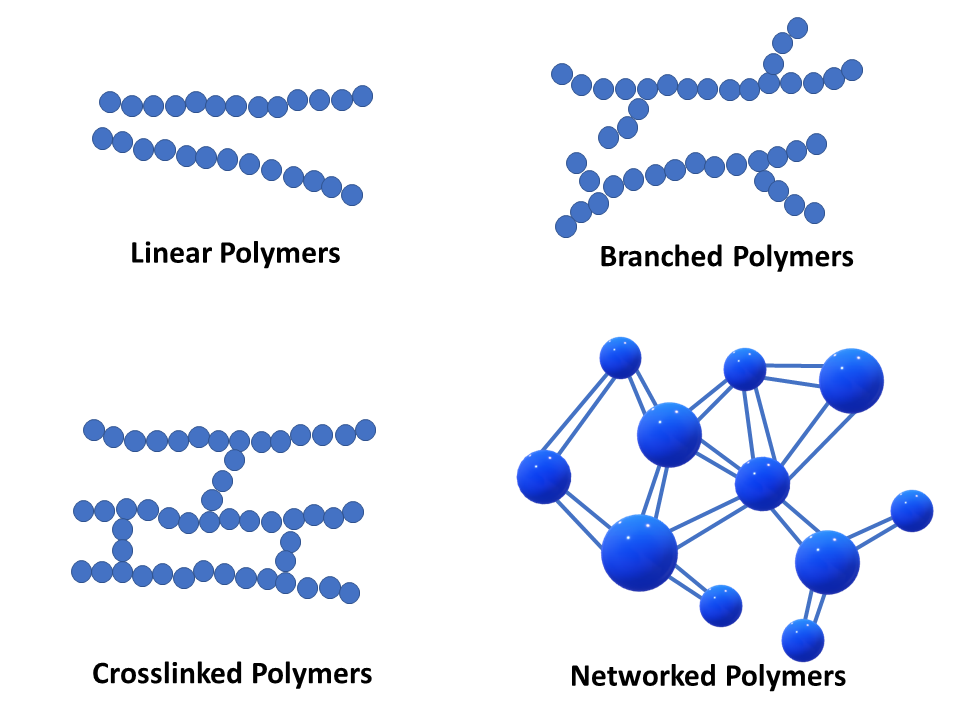
Polymers
Long chains of repeating units (monomers)
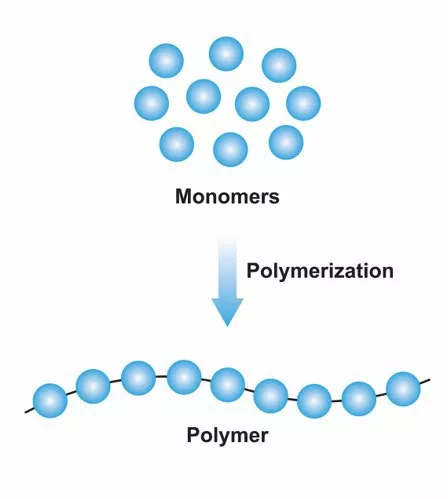
Monomers
Individual molecules that make up the polymer chains
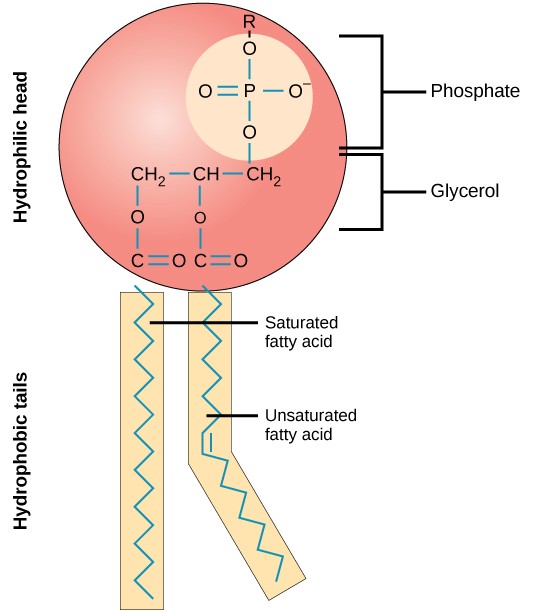
What are the parts of a fatty acid and what are their chemical properties?
The hydrophilic head
Polar group
Phosphate
Glycerol
Two hydrophobic fatty acid tails
One tail is a straight, saturated, hydrocarbon tail (max # of H atoms attached to carbons)
The other tail is a bent, unsaturated, hydrocarbon tail (one or more double bonds present in the hydrocarbon tail)
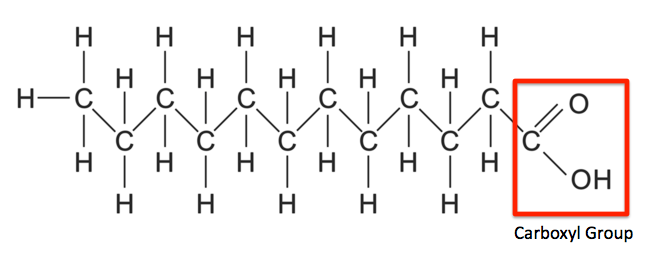
Carboxyl group
C double-bonded O
Hydrophilic
Molecules that interact with or dissolve in water
Hydrophobic
Molecules that DO NOT dissolve in or interact with water
Amphipathic
Molecules that have both hydrophobic and hydrophilic properties
Saturated fatty acid
Max number of hydrogen atoms attached to carbons (all single bonds)
Has simple straight hydrocarbon tails
Pack tightly and more likely to be solid at room temperature (high melting point)
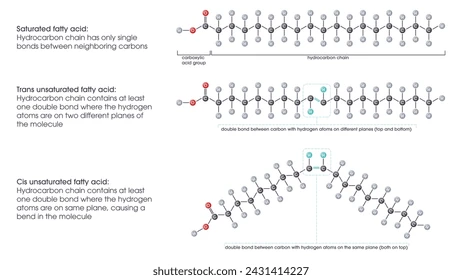
Unsaturated fatty acid
One or more double bonds in their hydrocarbon tail
Has a bent/kinked hydrocarbon tail
Pack loosely and more likely to be liquid at room temperature (low melting point)
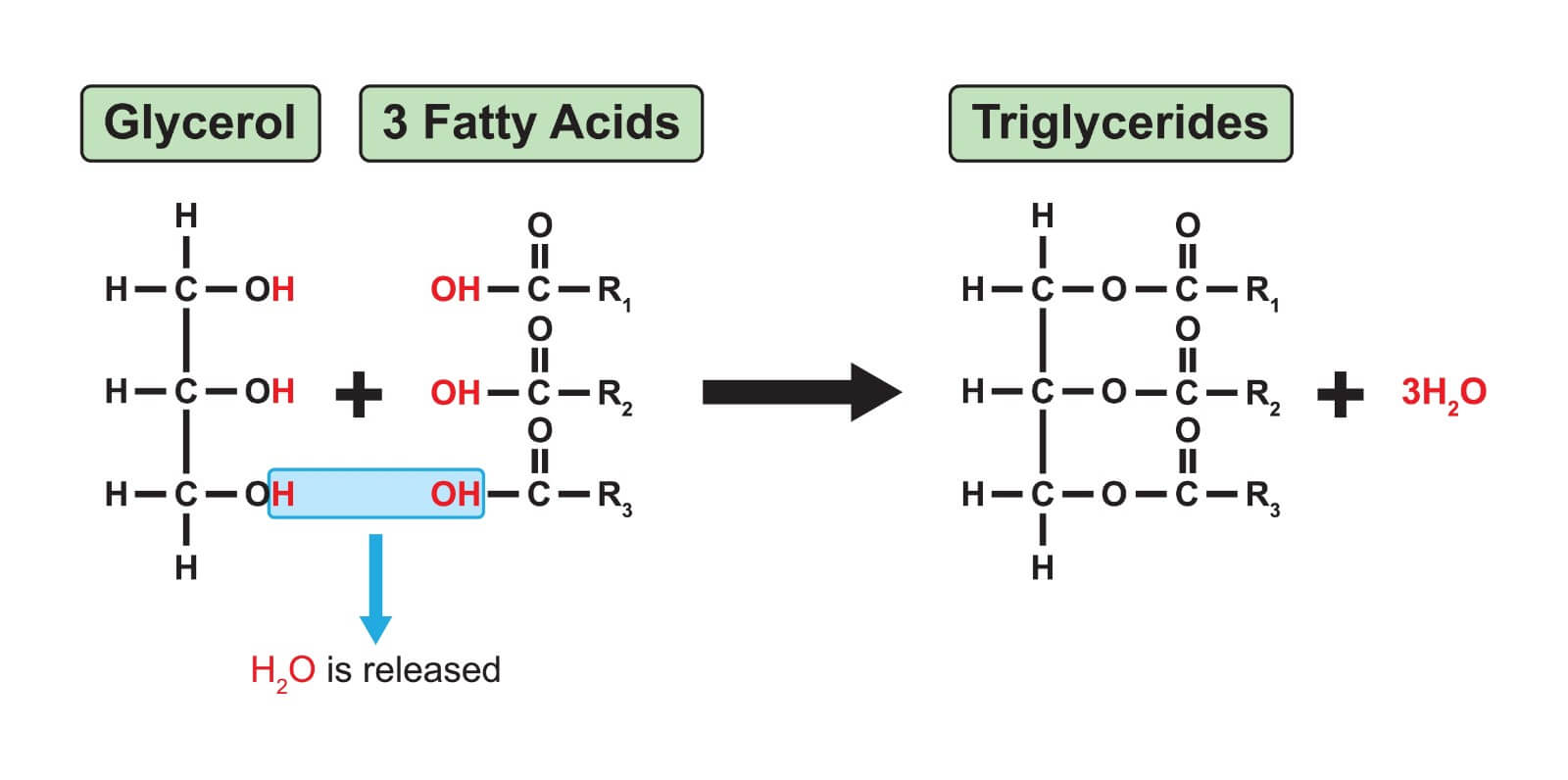
Triglycerides (Neutral fat)
Used to store energy
Made by condensation reactions
The head group of the fatty acids react with the hydroxyl group of glycerol (ester bond)
Three fatty acids will link to one molecule of glycerol to form a complete triglyceride (can be saturated or unsaturated)
Parts of phospholipids and their chemical properties
The hydrophilic head
Polar group
Phosphate
Glycerol
Two hydrophobic fatty acid tails
One tail is a straight, saturated, hydrocarbon tail (max # of H atoms attached to carbons)
The other tail is a bent, unsaturated, hydrocarbon tail (one or more double bonds present in the hydrocarbon tail)
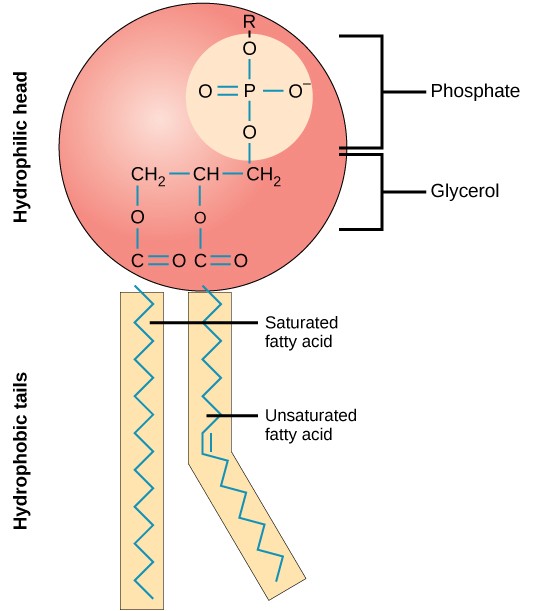
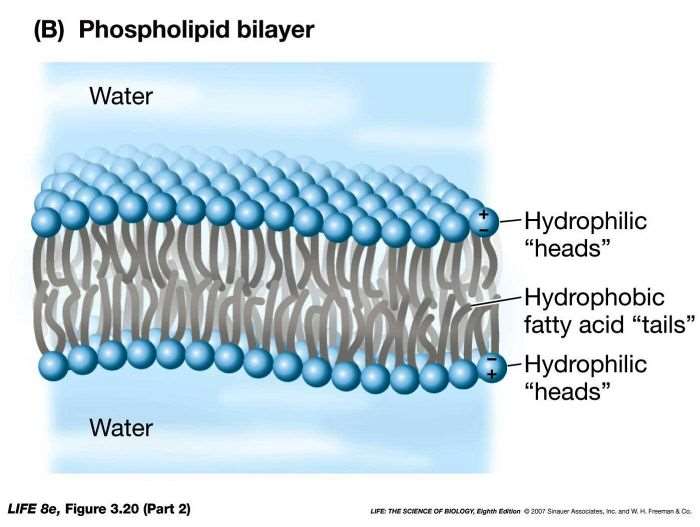
Hydrophobic effect
In a watery environment, the hydrophobic tails will clump together in a way that excludes water
Structure of lipid bilayer
Hydrophilic heads face the water
Hydrophobic fatty acid tails face each other and create the membrane barrier
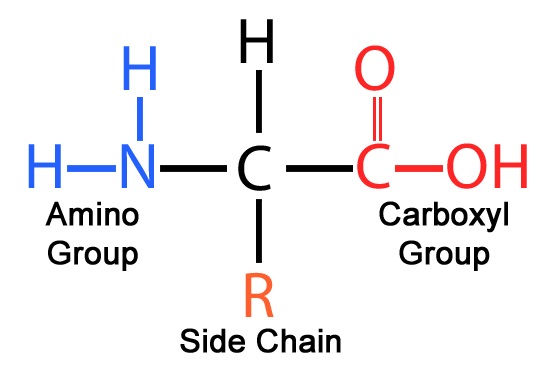
What are the four basic parts of an amino acid?
Central carbon/alpha carbon (central carbon that all other groups are attached to)
Amino group (basic)
Carboxyl group
R group or side chain (different for each type of amino acid)
What are the three major functions of amino acids in cells?
Used to make ATP in conditions of low available nutrients
Used to build signaling molecules (hormones and neurotransmitters)
Used to build polymers of amino acids (proteins)
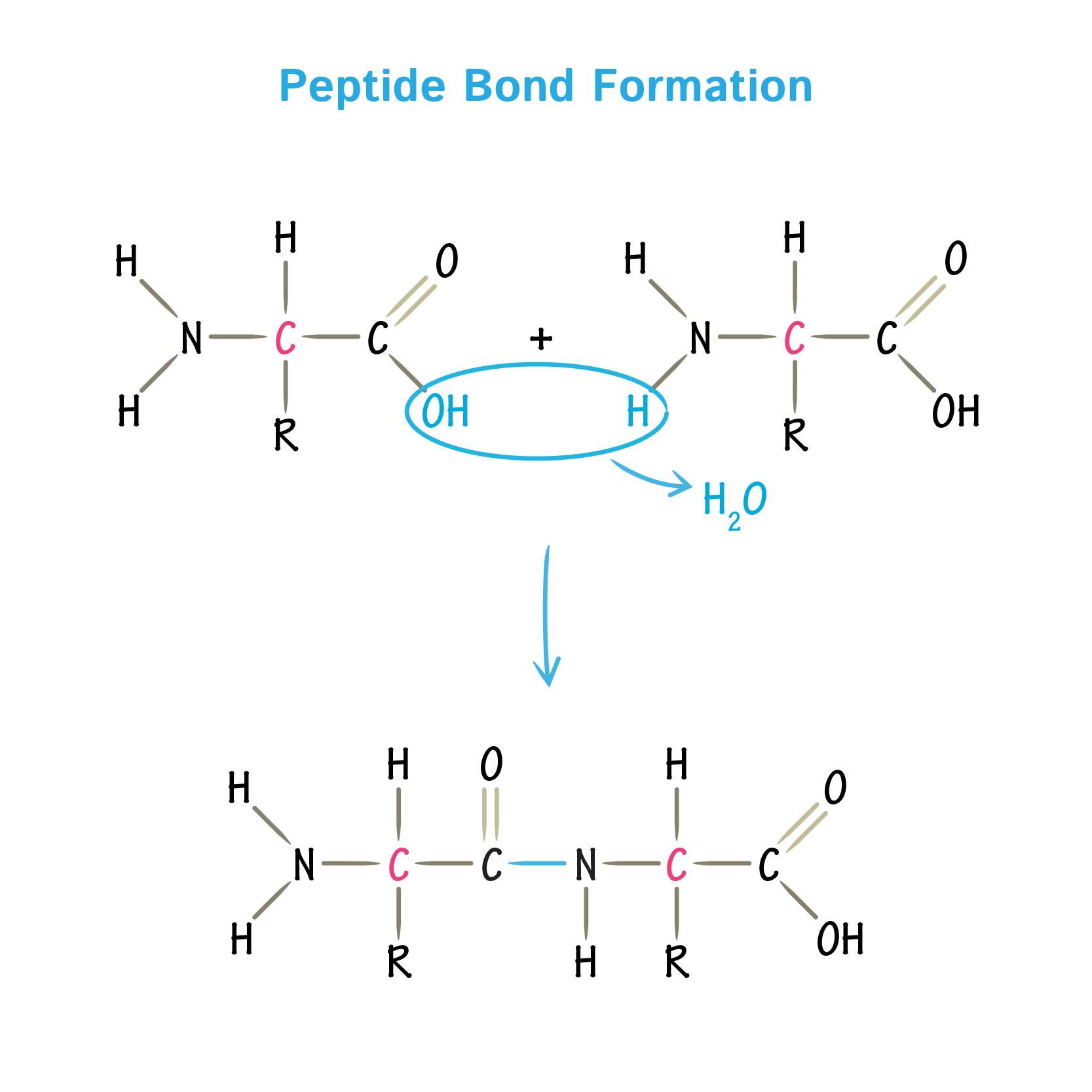
Peptide bond
Amino acids are chemically bound together into polymers by condensation reactions
The carboxyl of one amino acid reacts with the amino of the next
This bond is an imide bond but when an imide is formed between two amino acids, its a polypeptide bond
Nomenclature:
Short amino acid chains (less than 10) = peptide
Longer amino acid chains (more than 10) = polypeptide
(Proteins are polymers of hundreds of amino acids on average)
Peptide vs. Protein
Peptide:
Smaller than proteins (consist of between 2-50 amino acids)
Protein:
Larger than peptides (consist of 50 or more amino acids)
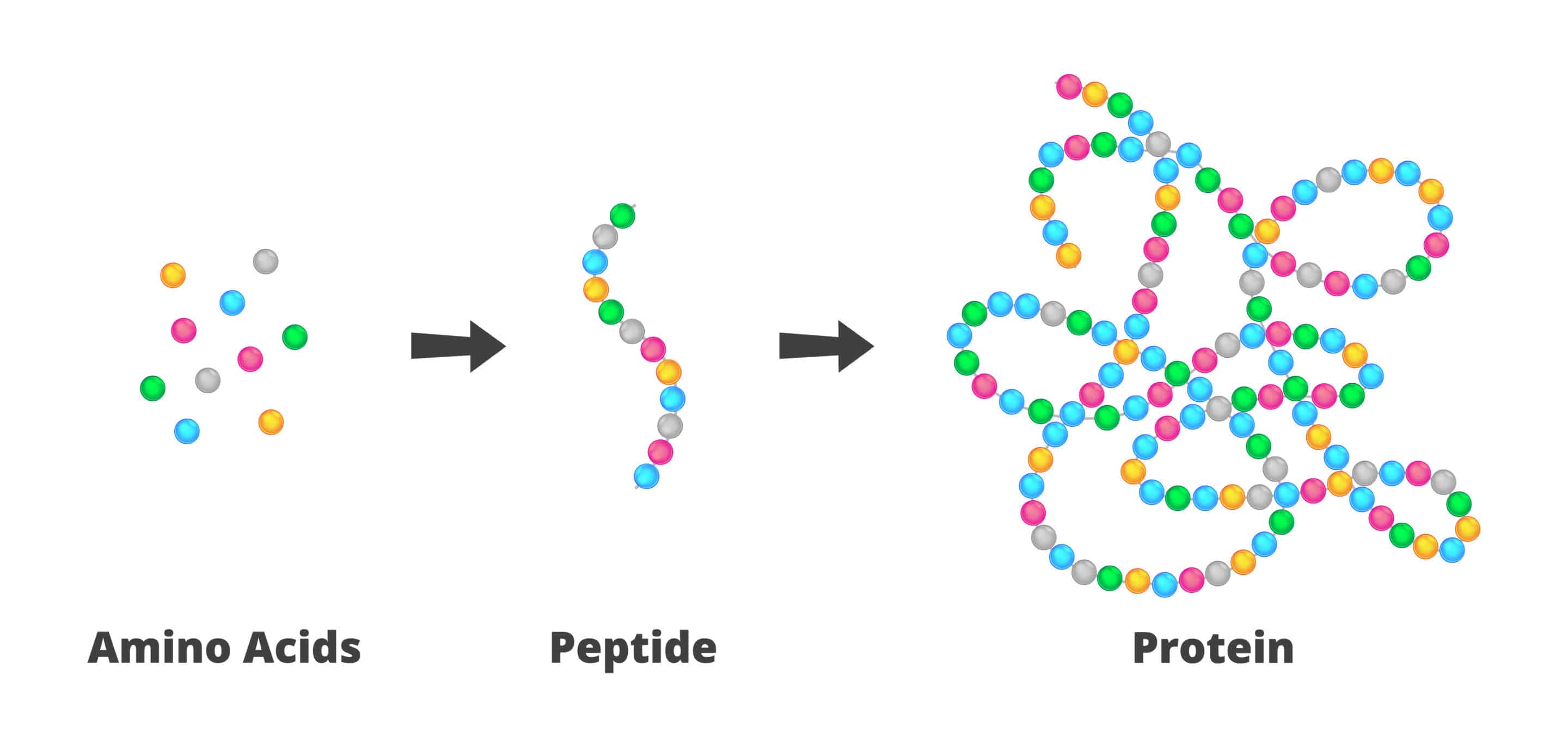
What are the three basic parts of a nucleotide?
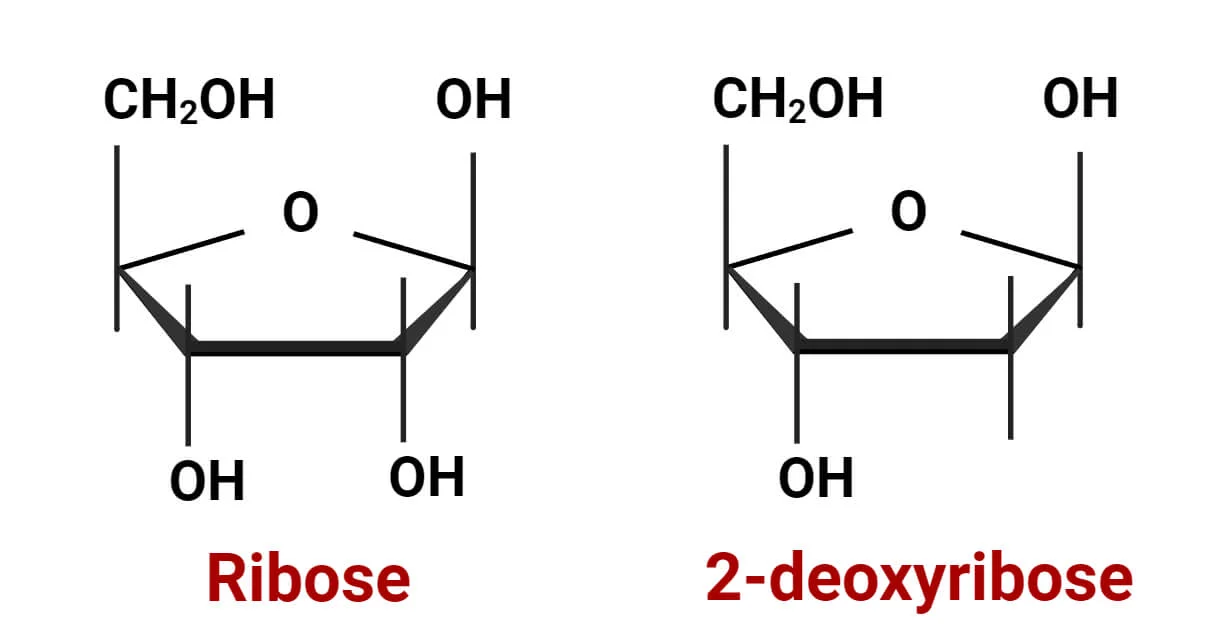
Pentose sugar
either ribose (RNA) or deoxyribose (DNA)
‘ = prime symbol (Ex: 1’ = 1 prime)
Phosphate group (PO43-)
also a functional group
Nitrogen-containing base
five different types
(polymers of nucleotides are called nucleic acids)
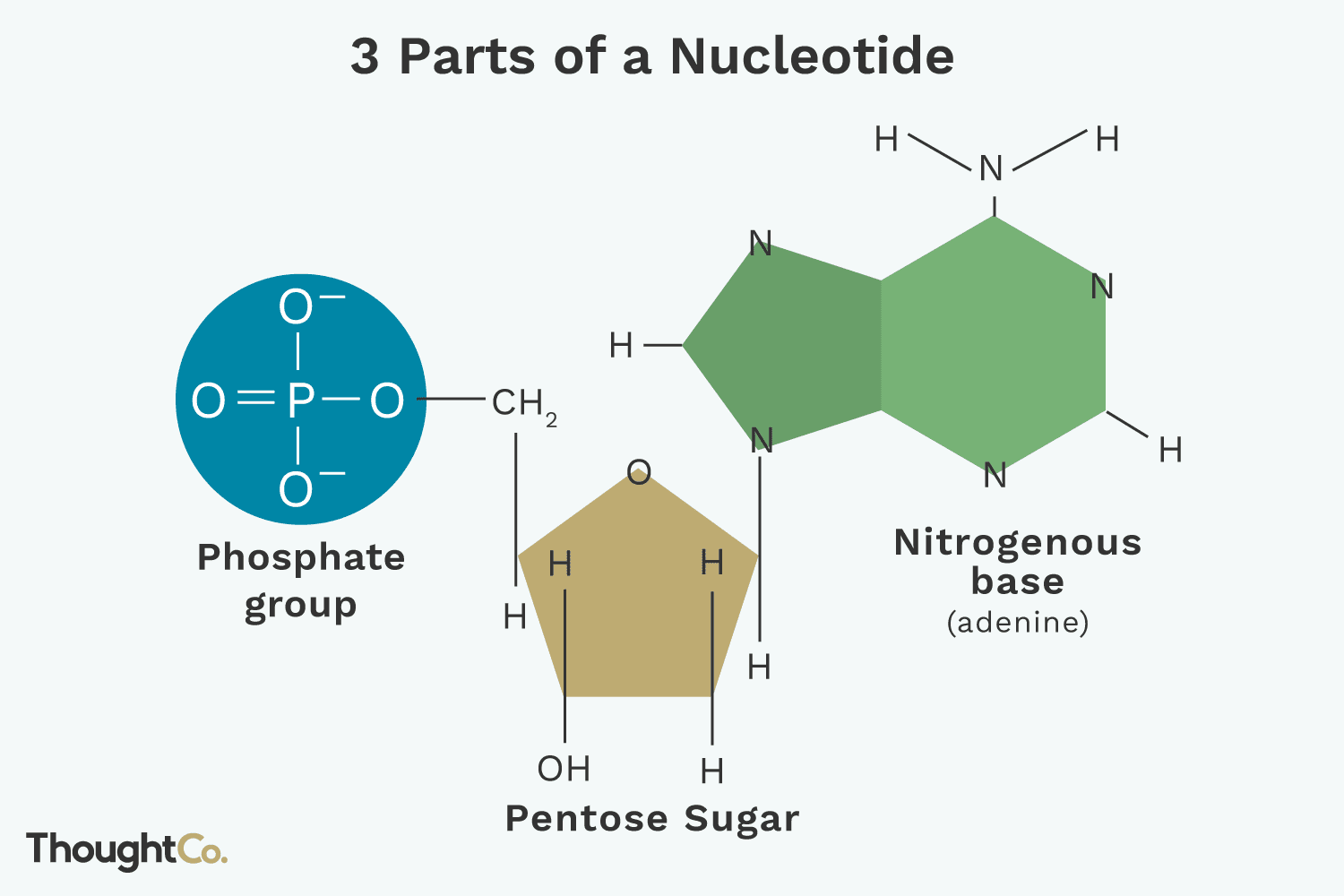
How does nucleotides form nucleic acids?
Nucleic acids are formed when nucleotides are covalently bonded together by condensation reactions
PO4 group attached to the 5’ carbon of one nucleotide chemically bonds to the hydroxyl group on the 3’ carbon on the next nucleotide
Because the nucleotides are asymmetrical, each end of the chain is different
PO4 (5’ end) + -OH (3’ end) + condensation reaction/dehydration synthesis = nucleic acid
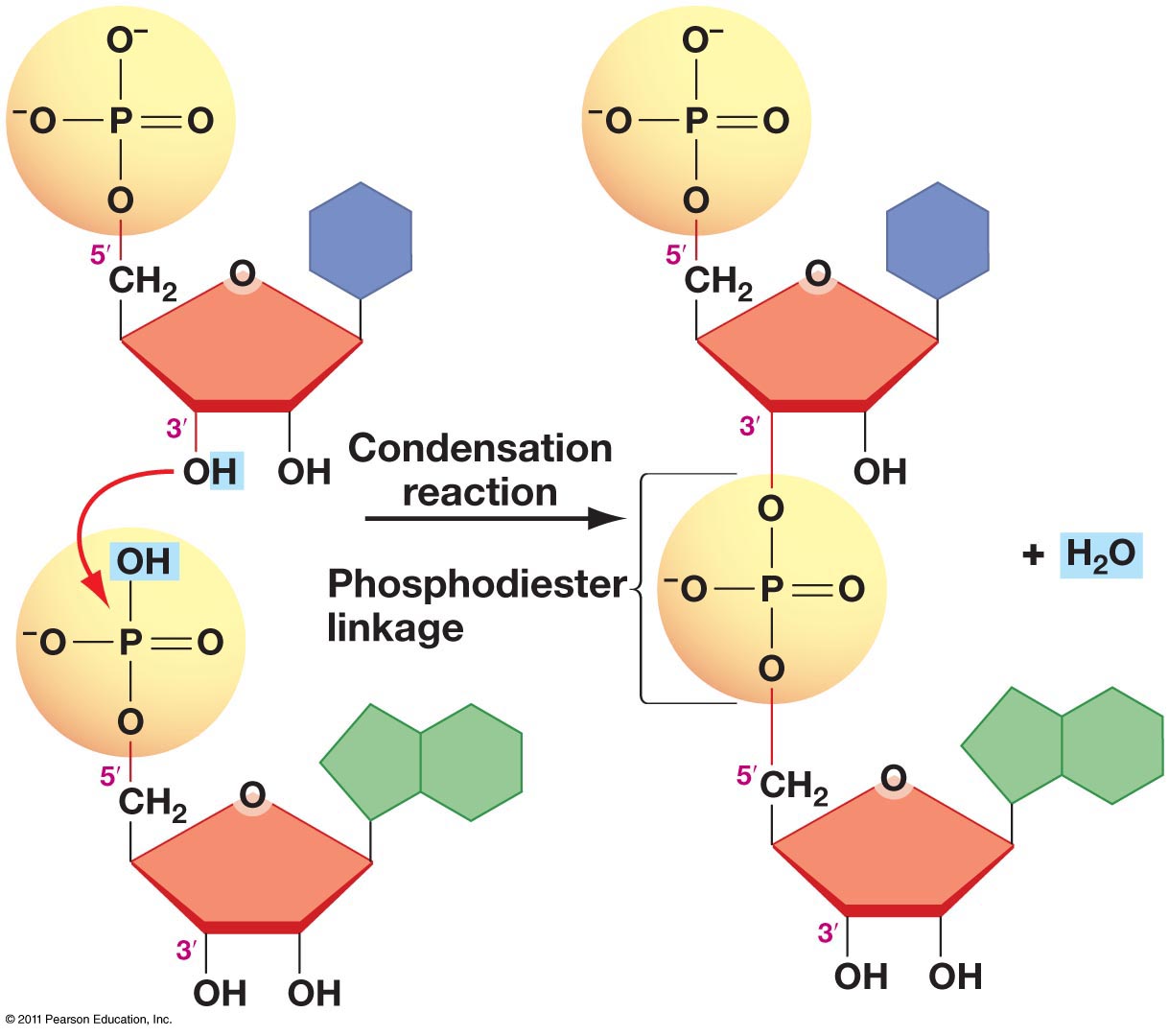
Phosphodiester bond
Result of PO4 group attaching to the 5’ carbon of one nucleotide chemically bonding to the hydroxyl group on the 3’ carbon on the next nucleotide (condensation reaction/dehydration synthesis)
“phospo-” beacuse the central atom is a Phosphate
“di-” because there are two ester groups
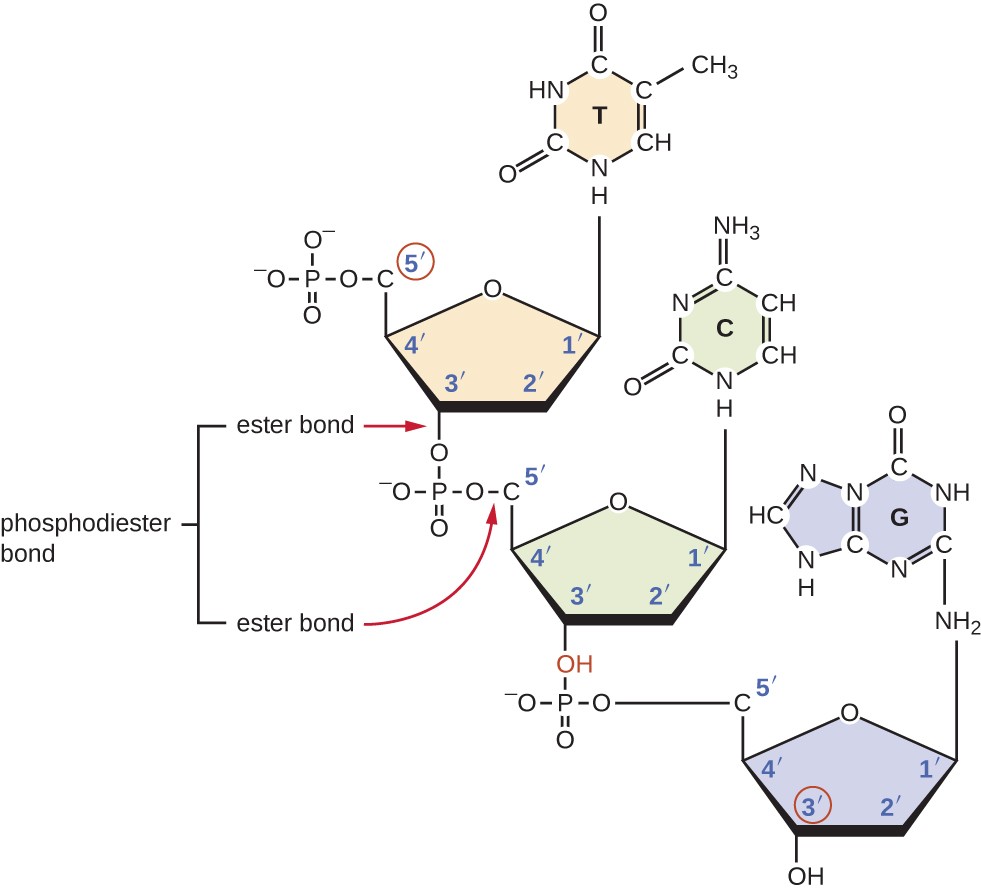
What are the five types of bases?
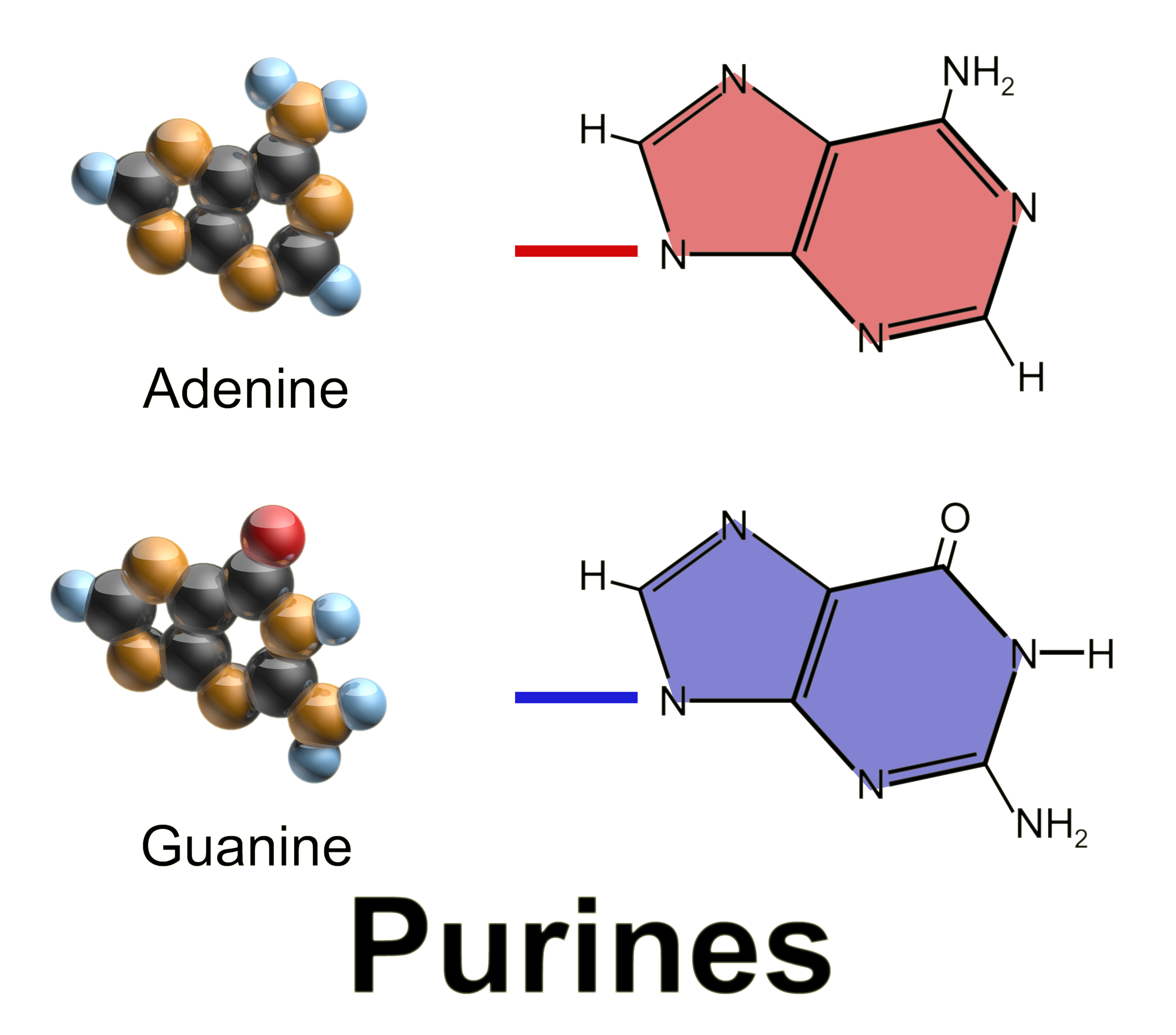
1. Adenine:
double ring structure and is a purine
2. Guanine:
double ring structure and is a purine
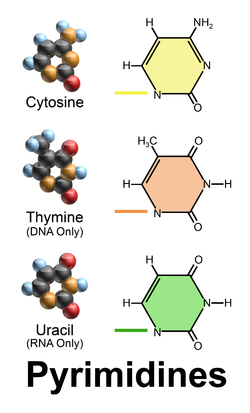
3. Uracil:
single ring structure and is a pyrimidine
4. Cytosine:
single ring structure and is a pyrimidine
5. Thymine:
single ring structure and is a pyrimidine
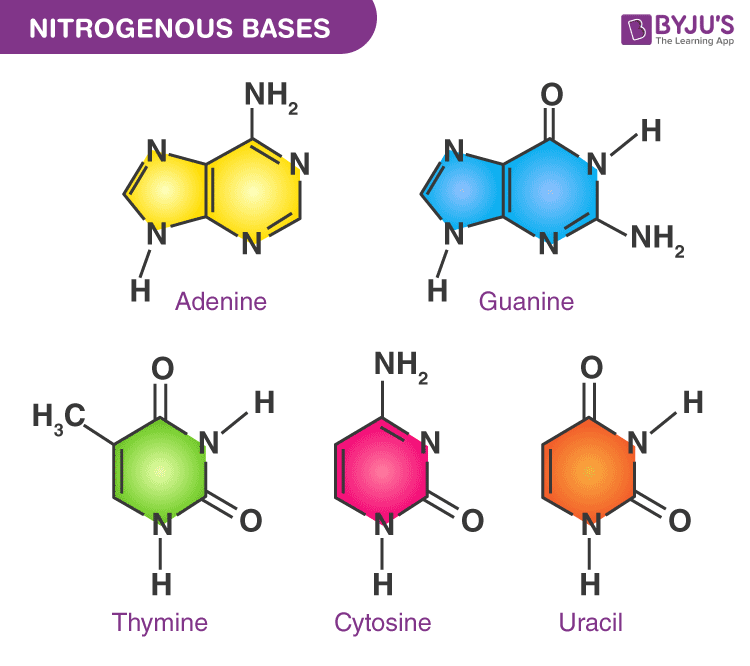
Purines
Bases that have a double ring structure:
Adenine
Guanine
Pyrimidines
Bases that have a single ring structure:
Uracil
Cytosine
Thymine
What are the two different types of pentose sugars found in nucleotides?
Ribose: (has hydroxyl groups on 2’ Carbon)
Deoxyribose: (missing a hydroxyl group on 2’ Carbon)
What are the major functions of nucleotides outside of their use in nucleic acids?
Energy storage
Catalysis
Cell signaling
Additional function #1 of nucleotides outside of their use in nucleic acid
Energy storage
Done by nucleotide triphosphate and diphosphate (Ex: ATP)
Diphosphate (2 phosphate groups covalently bonded to each other)
Triphosphate (3 phosphate groups covalently bonded to each other)
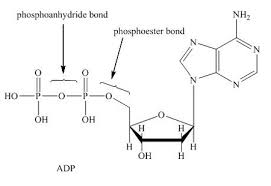
The bonds between the phosphate groups are called phosphoanhydrides
Energy is released when the bond is broken by hydrolysis reaction
Additional function #2 of nucleotides outside of their use in nucleic acid
Catalysis
Assists in speeding up a chemical reaction
Some enzymes require additional catalysts known as coenzymes (used in glycolysis and is derived from an adenine nucleotide)
Additional function #3 of nucleotides outside of their use in nucleic acid
Cell signaling
Cyclic nucleotides (where the phosphate is bonded to both the 5’ and 3’ hydroxyl) are important signaling molecules [Ex: cyclic AMP/cAMP]
Phosphoanhydride bond
Diphosphate (2 phosphate groups covalently bonded to each other)
Triphosphate (3 phosphate groups covalently bonded to each other)
The bonds between the phosphate groups are called phosphoanhydrides
Energy is released when the bond is broken by hydrolysis reaction
Nucleotide naming conventions
Nucleotides come in many forms so naming them accurately is important:
Three elements:
#1• Type of sugar:
If deoxyribose, add d if abbreviation and deoxy - if full name
If ribose, nothing additional required
#2• Name of the nucleoside:
- Nucleoside =
base + sugar
#3-Number of phosphates:
One: mono -
Two: du -
Three: tri-
Examples:
A deoyribose nucleotide with a guanine base and three phosphates (deoxyguanisine triphosphate/dGTP)
A ribose nucleotide with a cytosine base and two phosphates (cytadine diphosphate/CDP)
