1.5 Atomic and Nuclear Physics ☢️
1/82
Earn XP
Description and Tags
GCSE CCEA Specification GCSE Physics Double Award Science, Triple Award Science Unit 1: Motion, Force, Moments, Energy, Density, Kinetic Theory and Radioactivity
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
83 Terms
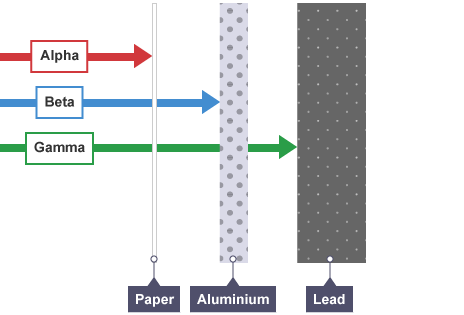
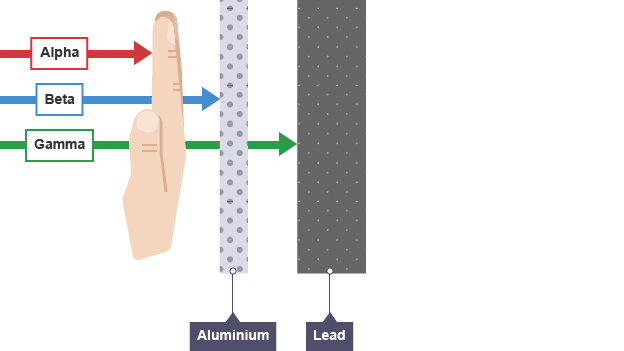
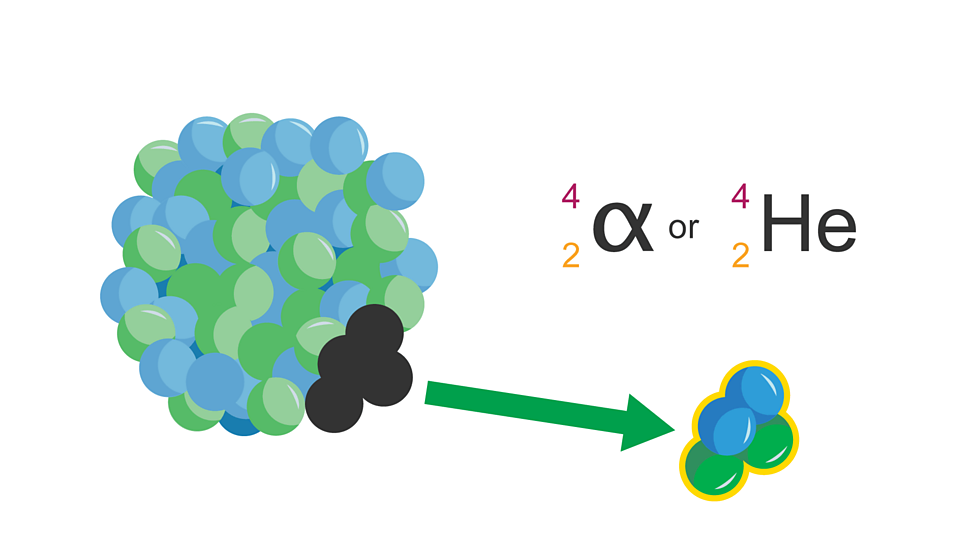
Alpha particle (α)
Helium nucleus of two protons/ neutrons
strong ionising power
weak penetrating power (paper/ skin)
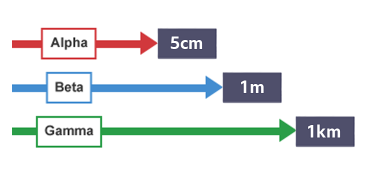
range of 5cm
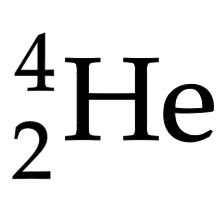
Beta particle (β)
neutron splits into proton and high speed electron which is ejected from nucleus
moderate ionising power
moderate penetrating power (few mm aluminium)
range of 1m
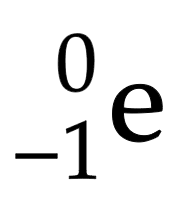
Gamma ray (γ)
Electromagnetic wave used to ‘relax’ an unstable nucleus
weak ionising power
strong penetrating power stopped by several cm lead or 1m concrete
range of 1km
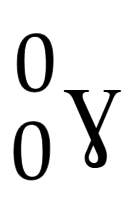
Ionising power
ability to ionise materials by displacing electrons, greater charge will ionise more
Penetrating power
ability to pass through matter, greater mass will penetrate less
Range (in air)
How far radiation travels in air before absorbed
Nuclear reactor
equipment in nuclear power station which fission (or fusion) takes place
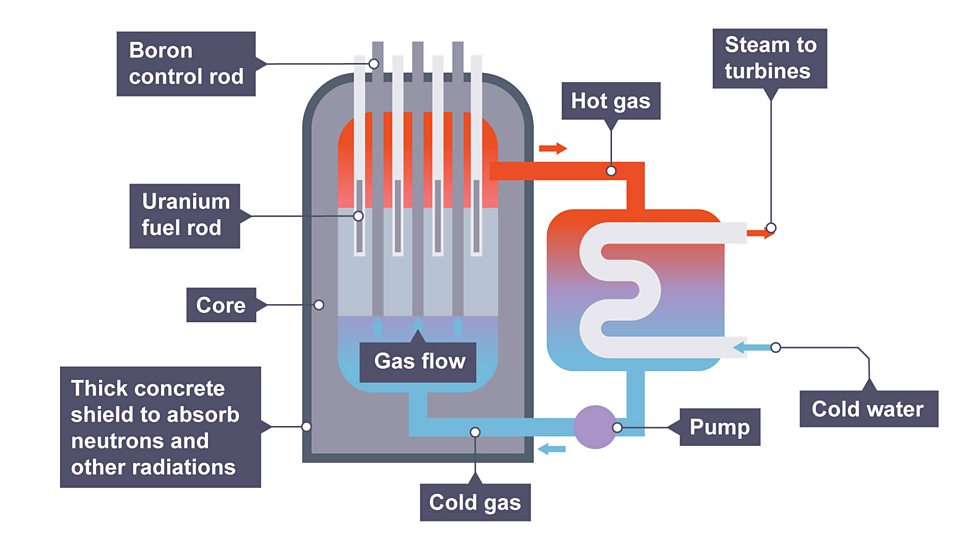
How does nuclear reactor work
neutrons are absorbed by control rods, heating water which can be transferred into electricity
Nuclear fuel for fission
uranium/ plutonium/ thorium isotopes (fissile material) obtained from mining ore
Nuclear fuel for fusion
hydrogen/ deuterium/ tritium found in oceans and seas
Unstable nuclei
has too few or too many neutrons
Radioactive decay
random process where an unstable nucleus emits radiation to become more stable
Types of nuclear radiation
Alpha particle, beta particle, gamma ray, neutron
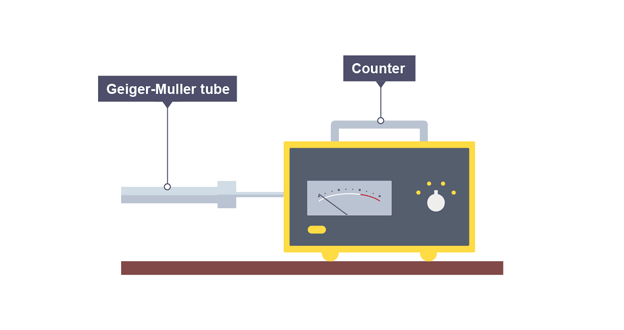
Geiger-Muller (GM) tube
detects ionising radiation and measures activity of radioactive source
activity
rate which a source of unstable nuclei decays
unit for activity
Becquerels (Bq)
count-rate
number of decays recorded each second by a detector
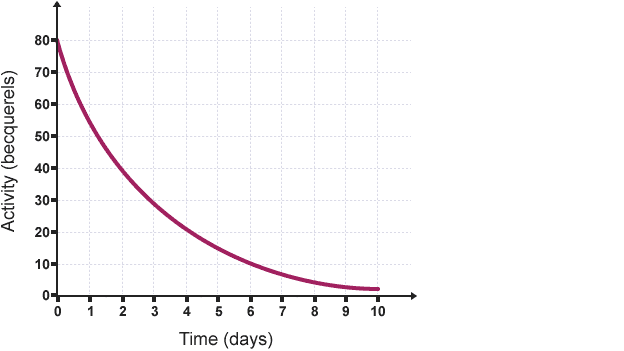
Half life
the time it takes for half the atoms in a substance to decay
Nuclear fusion
joining of two light nuclei to form a heavier nucleus, releasing energy (gamma rays)
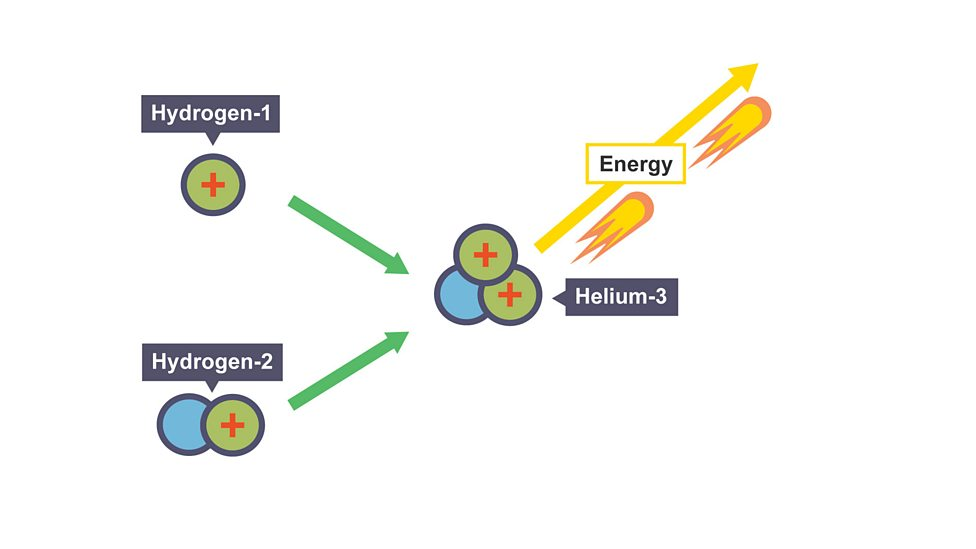
Spontaneous fusion
Occurs in stars when two hydrogen nuclei fuse to form helium nucleus
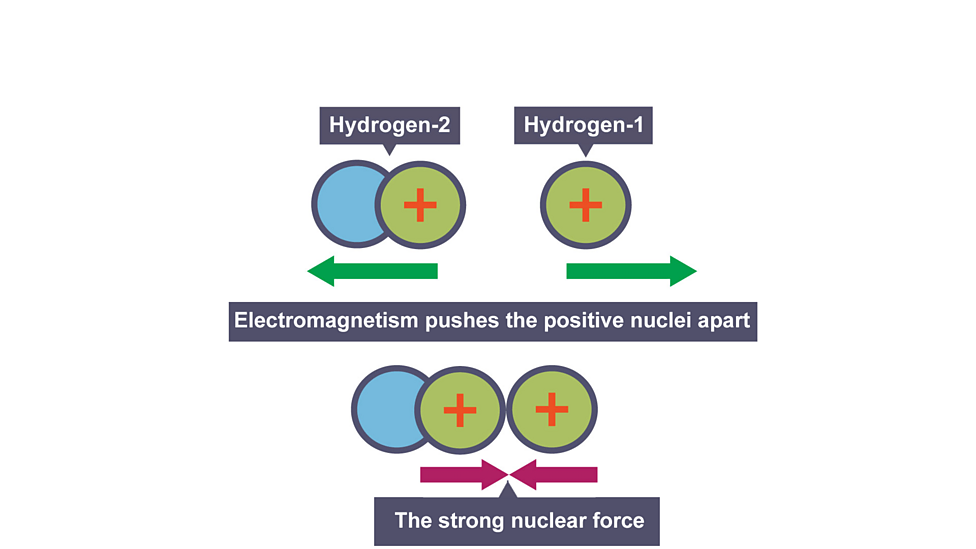
Conditions required for fusion
Very high temperatures and pressure to overcome repulsion
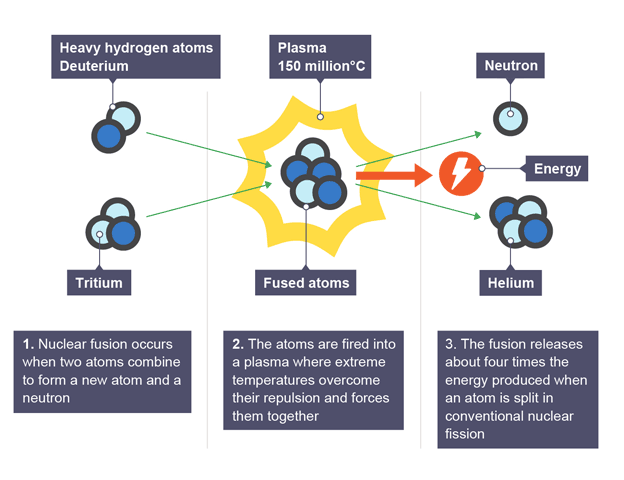
Fusion in the ITER project
deuterium and tritium are fired into plasma to overcome repulsion and fuses them together
releases four times the energy produced during conventional nuclear fission and helium
Mass-energy conversion
extra mass of fusing particles is converted into energy
repulsion
two nuclei repel each other as they are both positive
Advantages of fusion
solve world’s energy needs
hydrogen/ deuterium are widely available and relatively cheap (seas/oceans)
does not emit any greenhouse gases or have radioactive waste
four million times more energy per kg than burning fossil fuels
Disadvantages of fusion
Temperatures approaching the the sun are required which is very difficult to reach/ contain
may take 50 years before it becomes commericially available
expensive to build and operate because of technology and conditions required
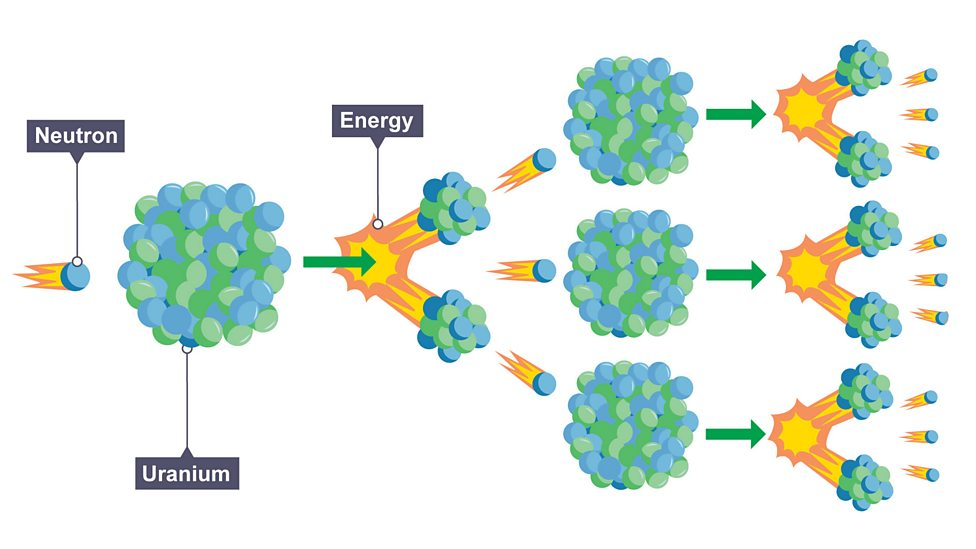
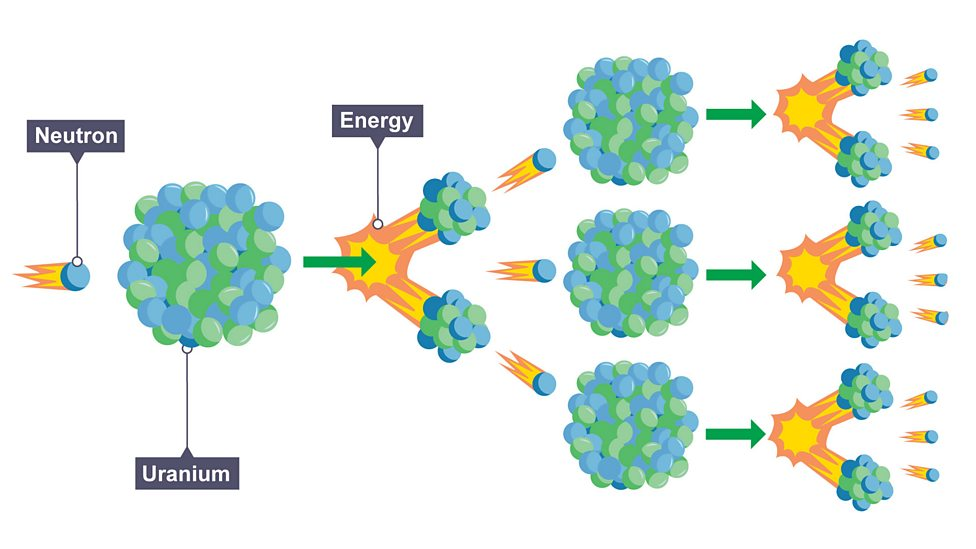
Nuclear fission
splitting of large and unstable nucleus, after absorbing slow moving neutron, producing two lighter nuclei which releases more neutrons and energy (gamma rays)
Fissile material
nuclei can be split by fission
e.g. uranium and plutonium
Spontaneous fission
spontaneous splitting of large and unstable nucleus (very rare)
Energy of fission products
products have kinetic energy so will move away
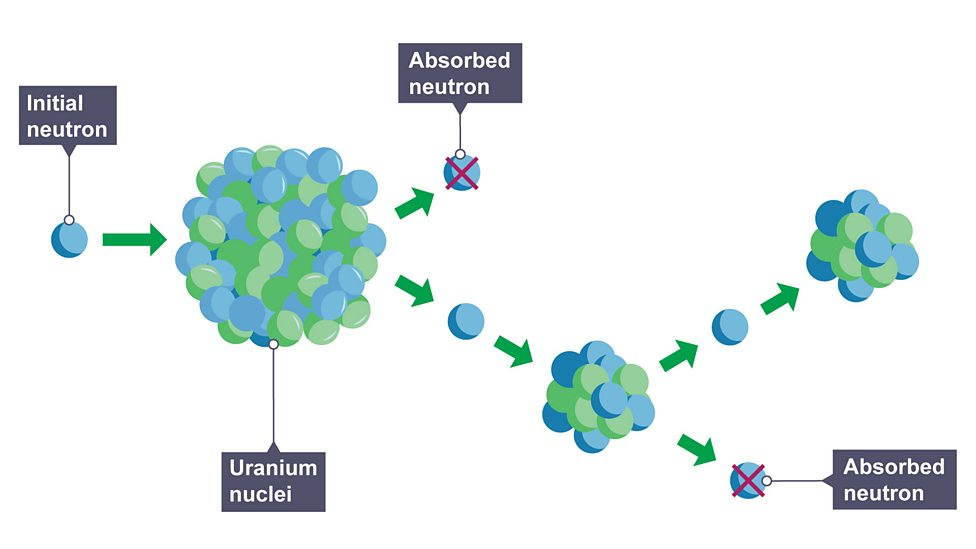
Chain reaction
neutrons released from nucleus cause splitting of further nuclei
Controlled chain reactions
rate of reaction limited to prevent getting out of control e.g nuclear reactors
Uncontrolled chain reaction
not limited and eventually leads to an explosion e.g atomic bomb
Advantages of fission
does not release the greenhouse gas carbon dioxide
releases one million times more energy per kg than burning fossil fuels
Modern reactor designs are extremely safe and create employment opportunities
Disadvantages of fission
incidents (Chernobyl) have caused huge damage to area surroundings
mining, transport and purification of fuels release lots of greenhouse gases and are non-renewable
radioactive waste is extremely dangerous and long lasting with fears of leaking out when stored
decommissioning is extremely expensive
Irradiation
object is exposed to radiation but doesn’t become radioactive
Sterilisation
using radiation to ensure a sample contains no living things
Using irradiation to sterilise food
gamma rays destroy any bacteria but do not affect the fruit itself, useful when not able to refrigerate
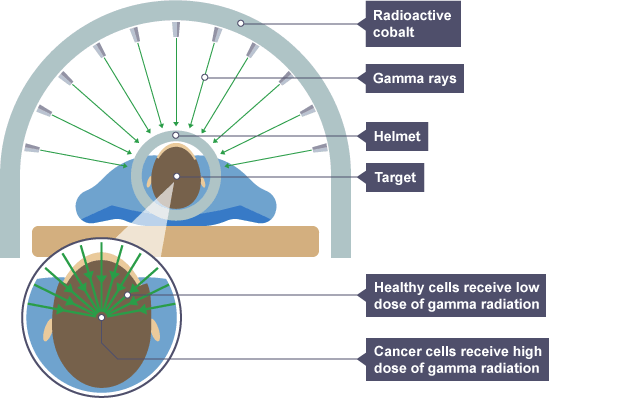
Using irradiation for medical purposes
sterilise surgical instruments without high temperatures and kill cancerous tumours using gamma rays (radiotherapy)
Radioactive contamination
unwanted radioactive atoms are mixed with other materials and they become contaminated
Why use tracers
find out what’s happening in an object without needing to break in
Using contamination for medical tracers
isotope is injected into body, then later passes out, where it is detected and used to form an image
Factors to consider when using medical tracers
gamma source so it can pass through body and be detected
short half life so no radioactive material is left
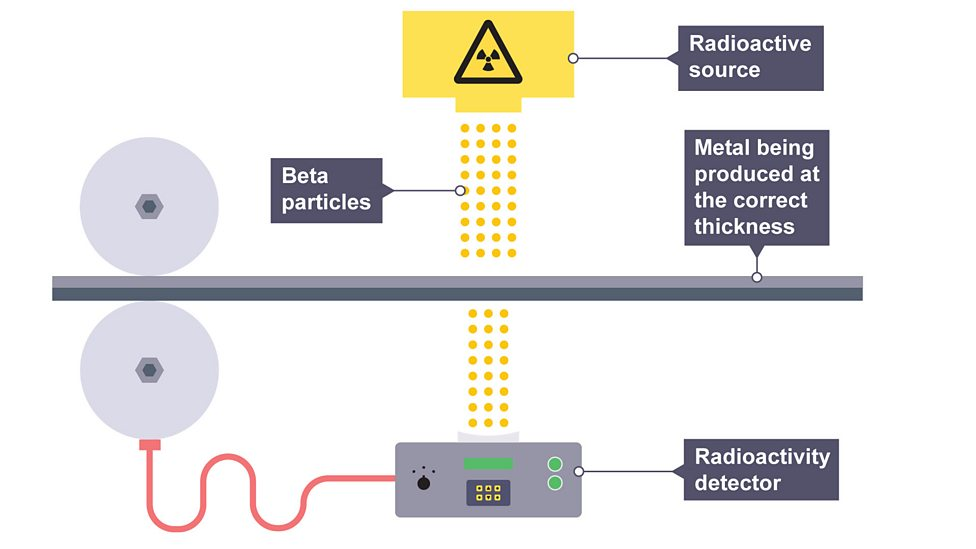
Using radiation for thickness control
emitter is placed on one side of a sheet and a detector on the other
if there is a change in thickness, the activity increases or decreases
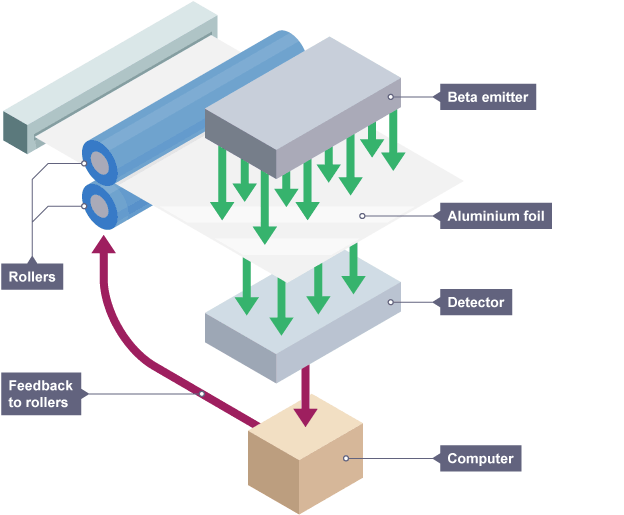
factors to consider for thickness control
beta source so it can penetrate and vary in activity
long half-life so count rate remains constant and not often replaced
Using contamination to check for leaks in water pipes
radioactive isotope is added to water and cracks cause contaminated water to leak
leaks have a build up of radiation which can be detected
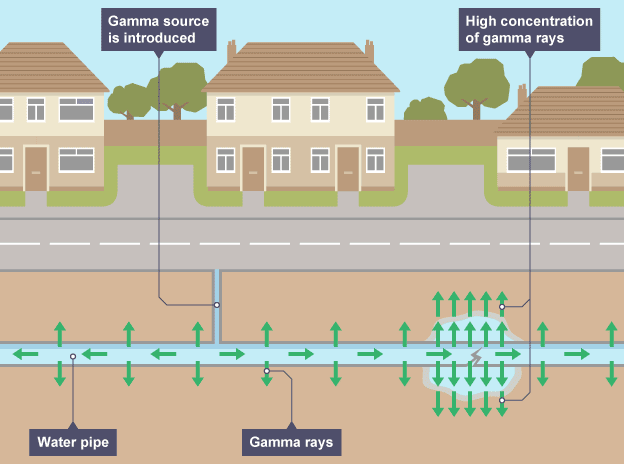
Factors to consider when checking for leaks
gamma source so it can penetrate ground
short half life so damaging effects don’t last long as water is poisonous
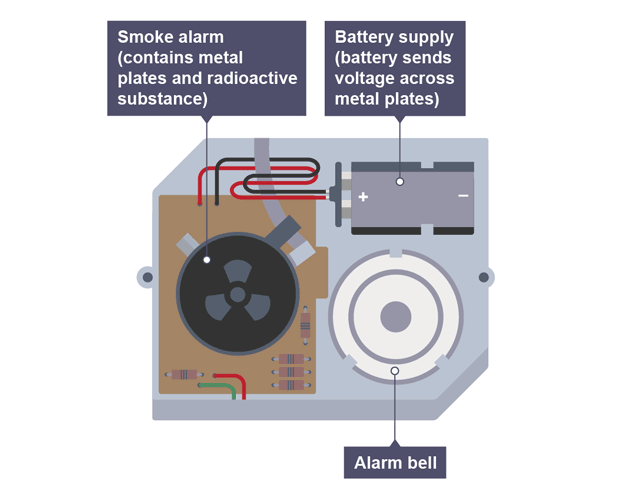
Using radiation for smoke detectors
particles pass between two charged metal plates, and ionise air, creating a current
if smoke enters particles are absorbed causing smaller currents to flow and alarm sounds
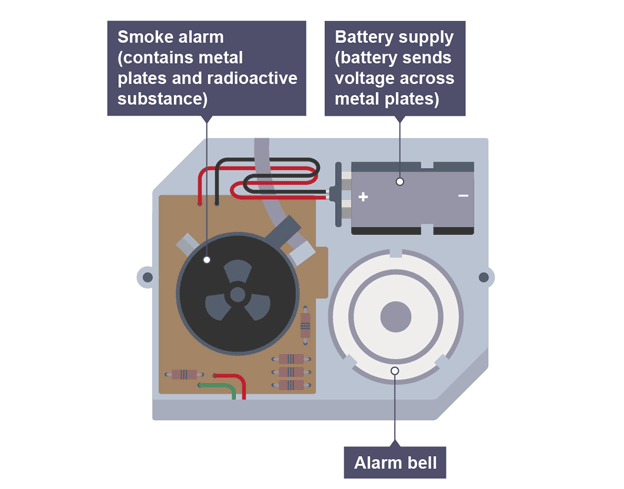
Factors to consider when using smoke detectors
alpha source so it ionises in air and doesn‘t penetrate far
long half-life so count rate remains constant and not often replaced
Dangers of irradiation and contamination
damages living cells and causes mutations
e.g radiation sickness or cancer
How to reduce irradiation
block with suitable shielding or as soon as source is removed
Why it is difficult to reduce contamination
radiation can’t be blocked and it is very difficult to remove it all
Precautions to take when using radioactive sources
sources kept in lead-lined box
wear protective clothing
avoid contact with bare skin
limit exposure time
use tongs to handle sources
monitor exposure
how can radiation be dangerous
causes ionisation, damaging genetic material which can make cells cancerous
large amounts of energy can damage or completely destroy cells
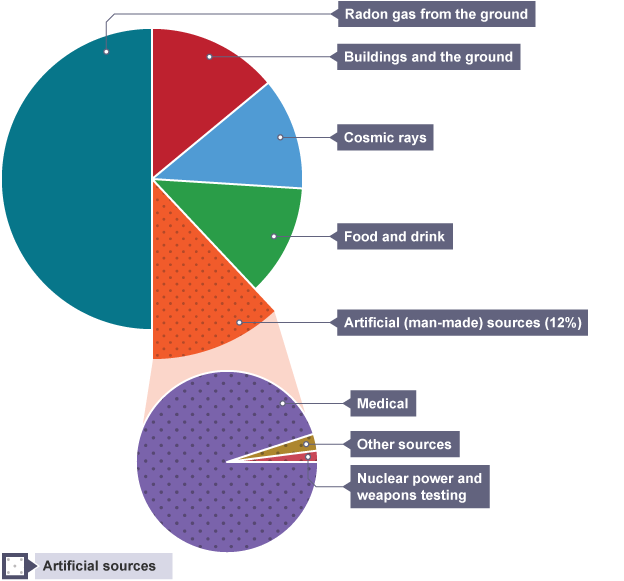
Background radiation
radiation around us all the time, even when no source is present e.g rocks or cosmic rays
Radiation in food chain
gas, living things and plants may absorb and pass it along
Human behaviour effect on background radiation
medical X-rays, radioactive waste power plants and radioactive fallout from nuclear weapons testing
Ways to reduce natural background radiation
high levels of radon gas require homes to be well ventilated to remove it
Why activity is unsuitable to measure radiation exposure
activity could be the same, but different decay, so would have a different effect
Why alpha radiation is more dangerous inside the body
more ionising and won’t penetrate skin so can’t escape from the body
Why beta radiation is more dangerous outside the body
less ionising but will penetrate the skin so can pass into the body
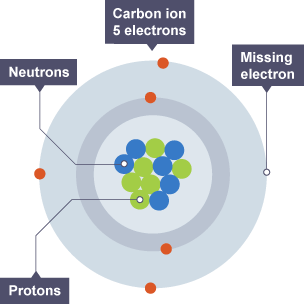
Atom
Building block of matter
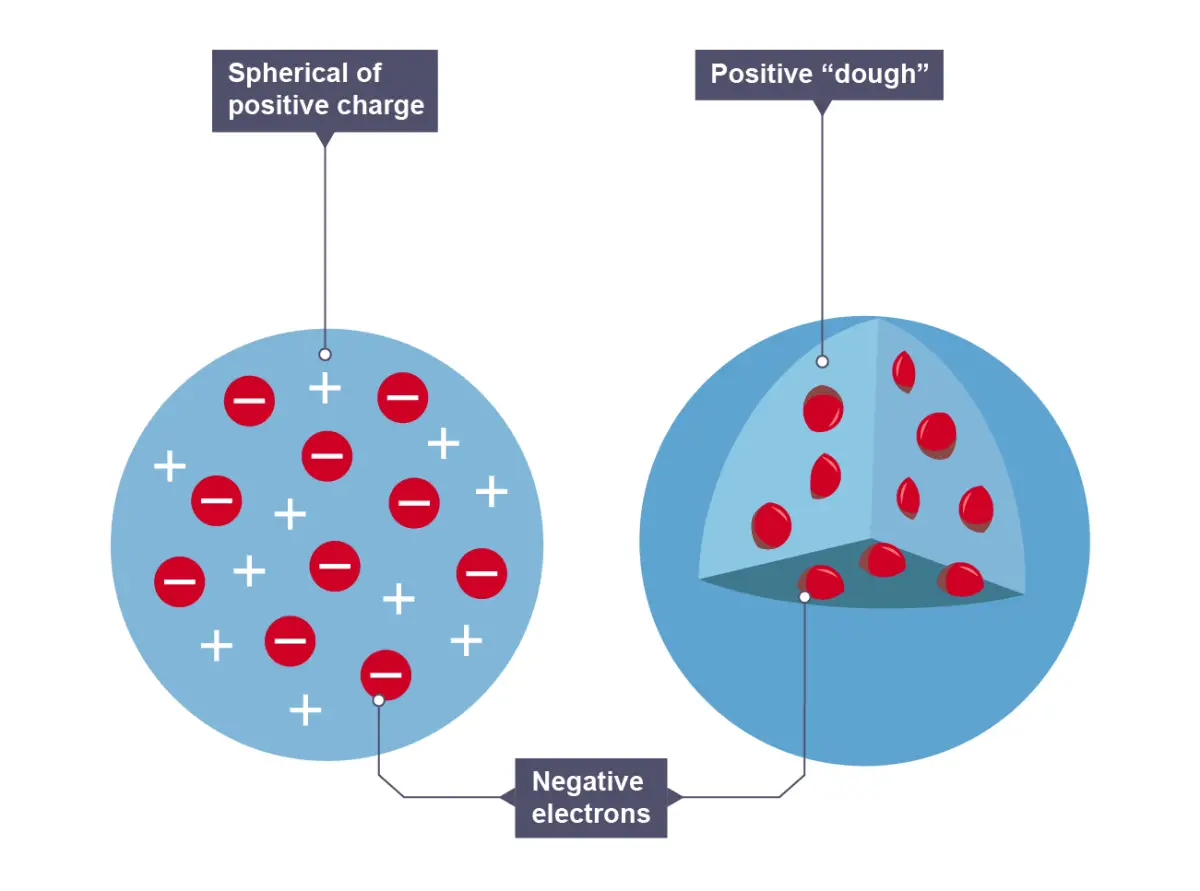
JJ Thomson
Discovered the electron and plum pudding model
Plum Pudding model (dough)
sphere of positive charge, with negatively charged electrons in it
Alpha particle scattering experiment
directed beam of alpha particles at very thin gold foil suspended in a vacuum, tiny flash of light is emitted when it hits the screen
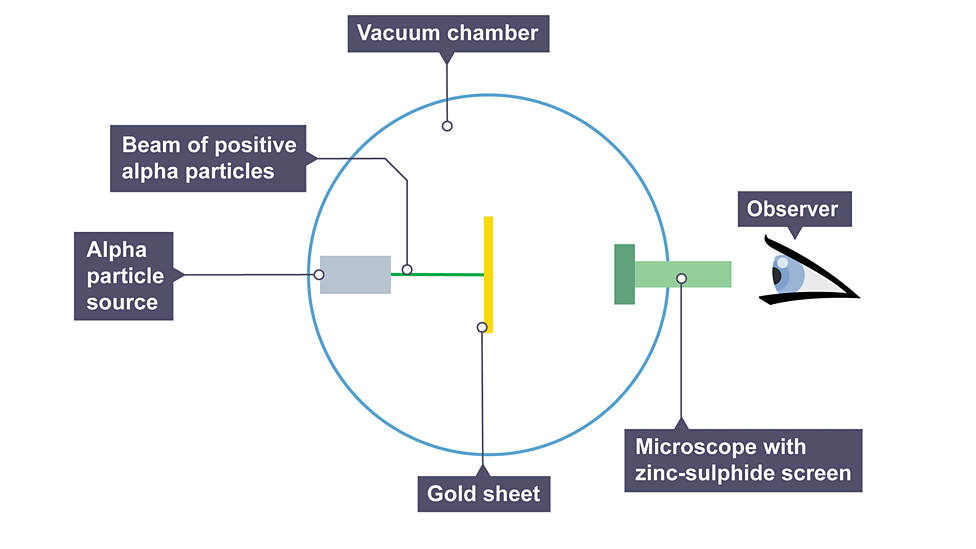
Observations from experiment
most alpha particles passed straight through foil but small number were deflected by large angles or straight back
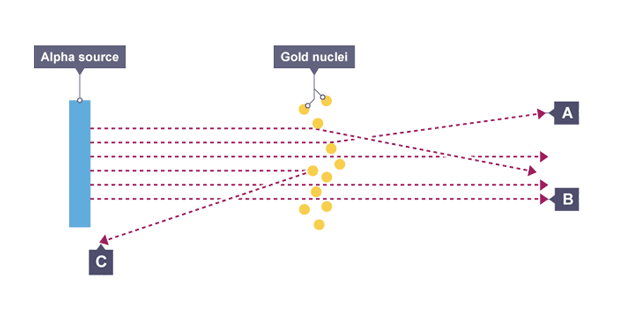
Conclusions from experiment
mass of atom is concentrated at the centre (nucleus) that had a positive charge
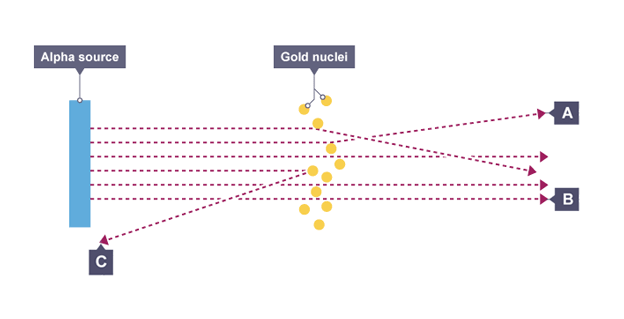
Nuclear model
atom is mostly empty space with positively charged centre containing most the mass and electrons orbiting
Ernst Rutherford
Tested plum pudding model to create nuclear model
Niels Bohr
Adapted nuclear model, suggesting electrons orbit at specific distances
James Chadwick
discovered the neutron
What happens to electrons when an atom absorbs energy
jump to higher levels (larger shells)
When electrons in an atom move to a lower energy level
atom emits energy as frequency of light
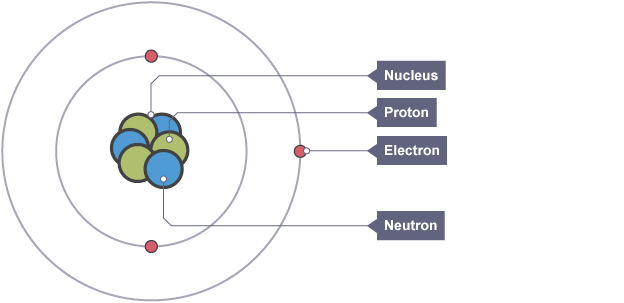
Structure of an atom
positively charged nucleus surrounded by electrons
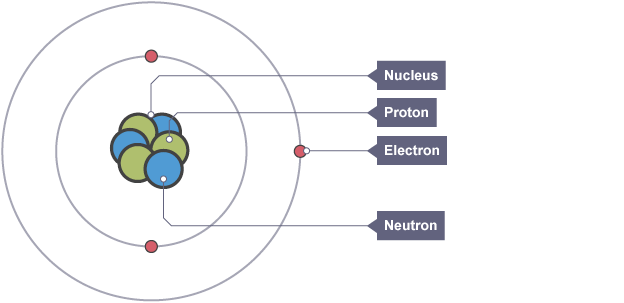
Proton
positively charged particle found in the nucleus which defines the atom
+1, 1
Neutron
neutral particle found in the nucleus
0, 1
Electron, charge and mass
negatively charged particle found orbiting the nucleus in shells
-1, 1/1840
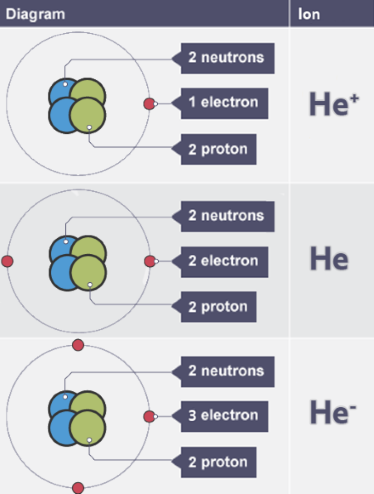
Ion
atom that has lost or gained electrons
Reason why atoms have no overall charge
number of electrons is equal to the number of protons so charges cancel out
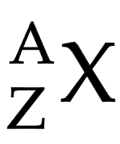
Atomic number (Z)
number of protons in the nucleus
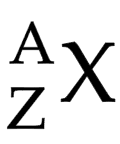
Mass number (A)
total number of protons and neutrons in the nucleus
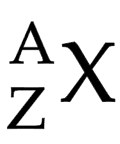
Symbol (X)
represents what element it is
Isotope
atoms of same element with same number protons but different number of neutrons