MICR5831 L12: Iron Acquisition Mechanisms 9/29/25
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
81 Terms
What is this?
-Central trace element in respiration (cytochromes, iron-sulphur proteins)
-Co-factor in many enzymes
Fe (Iron)
What effect will this have on bacteria?
-Iron deprivation
-Extreme iron deficiency
-Deprivation: bacteriostatic
-Deficiency: bactericidal
What is this?
-Uses Manganese instead of iron
-Causes Lyme disease
-No iron-dependent enzymes, survives iron absence
Borrelia burgdorferi
Which iron-binding protein is this?
-Found in Liver
-Serum protein responsible for iron transport in blood and tissue
Transferrin
Which iron-binding protein is this?
-Protein secreted at mucosal surfaces
-Found in saliva and tears
Lactoferrin
Which iron-binding protein is this?
-Contains heme
-70% of total body iron found in RBCs
Hemoglobin
Which iron-binding protein is this?
-Major protein used for intracellular iron storage
-Udhaya is lacking this intracellular
Ferritin
What is the level of Fe3+ in plasma?
>10uM
What is the level of free iron in plasma?
6E-9 μM
Why is the level of free iron in the plasma so much lower than the level of Fe3+ in the plasma?
-Free iron has lower solubility and protein binding
-Too little for bacterial growth
Which iron-capturing mechanism is this?
-Low molecular weight compounds
-Chelate iron with very high affinity
-Widespread, best studied mechanism
Siderophores
Which iron-capturing mechanism is this?
-Lactoferrin and Transferrin act as iron sources
Siderophores
Direct Contact
What are the two main chemical types of Siderophores?
1) Catechols
2) Hydroxamates
What type of siderophore is this?
-Aerobactin (E. coli)
Hydroxamate
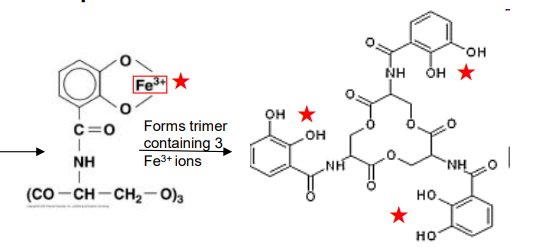
What type of siderophore is this?
-Enterobactin (E. coli)
-Forms trimer containing 3 Fe+ ions
Catechol
What is the first step of the Siderophore mechanism?
Secretion of siderophore into medium and tissues
What happens during the Siderophore mechanism after this?
-Secretion of siderophore into medium and tissues
-Complexes with Fe3+
-Removes Fe3+ from host protein
-Siderophore has higher affinity for iron
What happens during the Siderophore mechanism after this?
-Siderophore complexes with Fe3+ and removes it from host protein
-Iron-siderophore complex is taken into bacterial cell
-Specific transport mechanism
What happens during the Siderophore mechanism after this?
-Fe3+ Iron-siderophore complex is taken into bacterial cell
Iron is released from complex as Fe2+ by ferric reductase
What happens to reusable siderophores after it forms a complex with Fe3+ iron?
Ferric reductase lowers affinity, releasing Fe2+
What is this?
-Reduces Fe3+ to Fe2+
-Reduces iron affinity for reusable siderophores
Ferric reductase
What happens to nonreusable siderophores after it forms a complex with iron?
Siderophore is degraded by protease to release iron
What happens to the siderophore/membrane transport proteins when iron is low or high?
Low Iron: Synthesized
High Iron: Repressed
Why aren't siderophores and membrane transport proteins constitutively expressed?
-Expensive to produce
-Uses up building proteins and ATP
What is this?
-Repressor protein
-High levels of Fe2+ bind to it
-Represses transcription of siderophore synthesis/transport proteins
Fur (ferric uptake regulator)
Where does ferric enterobactin (FeEnt) complex get the energy to cross the Outer Membrane if ATP can only be found in the cytoplasm?
Proton Motive Force (PMF)
What is this?
-Helps FeEnt reach the Outer Membrane from the Inner Membrane
-Receives energy from PMF
-Assisted by ExbBD protein
TonB
What is this?
-Rotation is powered by PMF
-Promotes OM transporter to change conformation
-Allows FeEnt to pass through ABC transporter into cell
TonB
What happens if the following bacteria lose their ability to produce siderophores?
-E. coli, Pseudomonas, Yersinia mutagenesis
Loss of virulence
What happens if the following bacteria lose their ability to produce siderophores?
-Vibrio cholerae
-No difference
-Mutants are still virulent
Why does removing siderophores have no effect on Vibrio cholerae virulence?
-Ferrous iron can diffuse across Outer Membrane using porin channels
-Ferric iron is taken up without siderophore
-Pirates/imports enterochelin from other bacteria
True or False: Non-pathogenic bacteria do not produce siderophores
False
Why produce more than one siderophore?
-Some are more costly to produce
-Some have a higher affinity for iron
What Siderophore is this?
-Induction: Rapid (early stages)
-Fe Affinity: Low
-Reusable
Aerobactin
What Siderophore is this?
-Induction: Slow
-Fe Affinity: High
-Not reusable, degraded
Enterobactin
How can siderophores be exploited to deliver drugs to bacteria?
Antibiotics are covalently linked to siderophores
How does the antibiotic gain entry to the cell?
-Uses siderophore as Trojan horse
-Bypass the Gram Negative Outer Membrane
Which antibiotics are most successful at exploiting siderophores to cross bacterial Outer Membranes?
-Cephalosporins
-Siderophore-antibiotic hybrid has higher activity
-Protected against some β-lactamases
What is this?
-Antibiotic that acts on Beta lactamase-producing Gram Negatives such as K. pneumoniae
-Used to treat UTIs
Cefiderocol
Which iron-capturing mechanism is this?
-Bacteria bind directly to host transferrin/lactoferrin
-Use specific receptors for transferrin/lactoferrin
-Requires many membrane proteins to uptake Fe
Direct Contact
Which Direct Contact for iron uptake is this?
-Human pathogen, binds human transferrin in the liver
Hemophilus influenzae
Which Direct Contact for iron uptake is this?
-Bovine pathogen, binds bovine transferrin
Hemophilus somnus
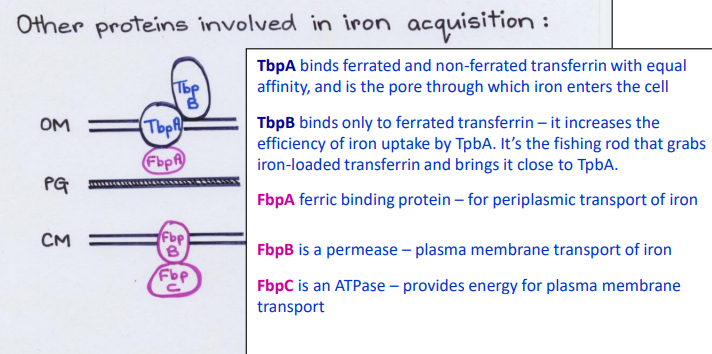
What Direct Contact protein is this?
-Binds ferrated and non-ferrated transferrin with equal affinity in the Outer Membrane
-Pore channel that allows iron to enter cell from outside
TbpA
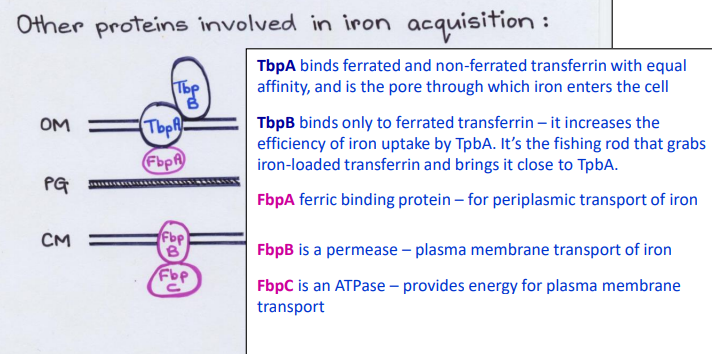
What Direct Contact protein is this?
-Binds only to ferrated transferrin in the Outer Membrane
-Increases the efficiency of iron uptake by TbpA pore
TbpB
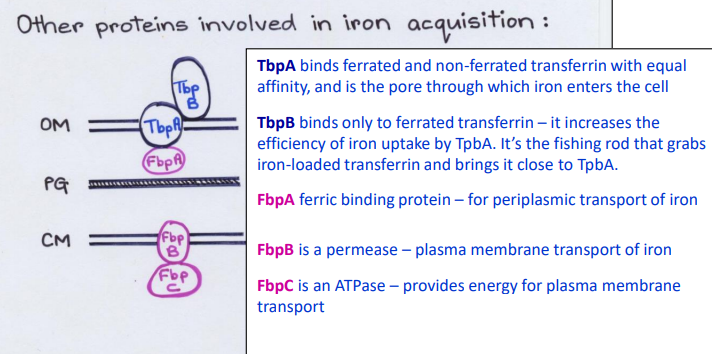
What Direct Contact protein is this?
-Fishing rod that grabs iron-loaded transferrin and brings it close to TbpA
TbpB
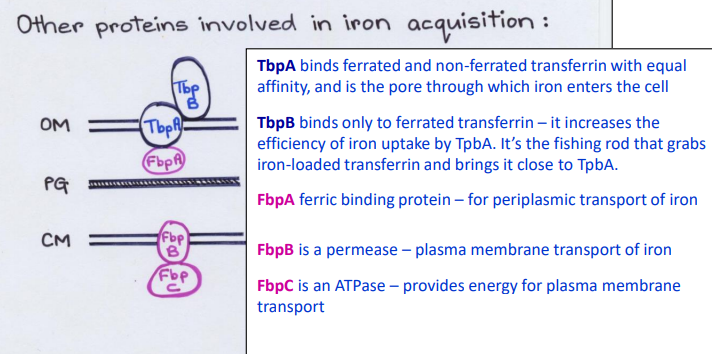
What Direct Contact protein is this?
-Ferric binding protein in the periplasm
-Periplasmic transport of iron
FbpA
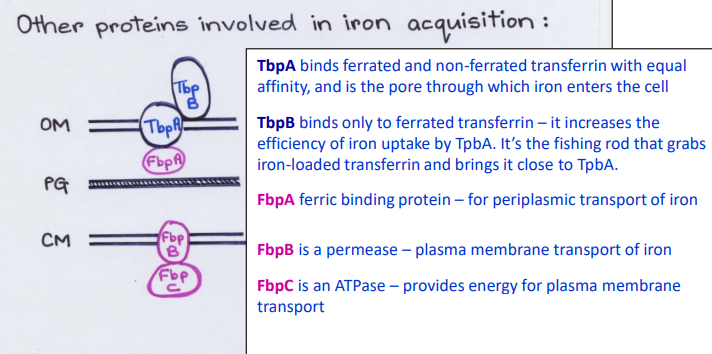
What Direct Contact protein is this?
-Permease channel/carrier
-Plasma membrane transport of iron
FpbB
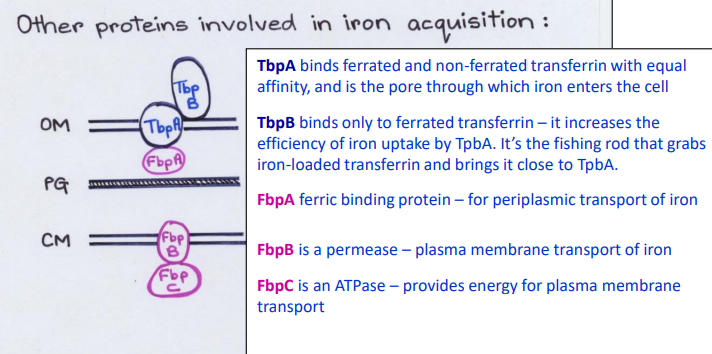
What Direct Contact protein is this?
-ATPase
-Provides energy for plasma membrane transport
FbpC
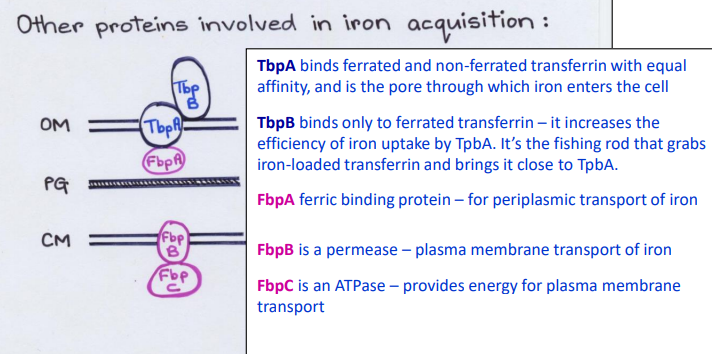
List the Direct Contact proteins in the Outer Membrane (Top-> bottom)
1) TbpB
2) TbpA
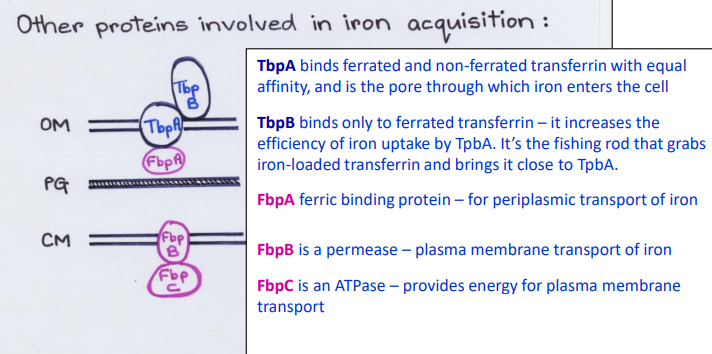
List the proteins in the Periplasm
FpbA
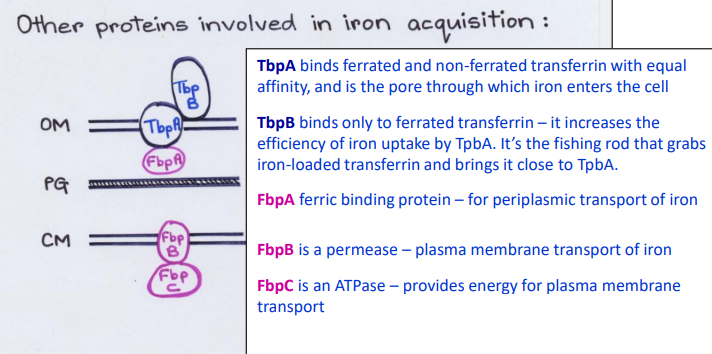
List the Direct Contact proteins in the Inner Membrane (Top-> bottom)
1) FbpB
2) FbpC
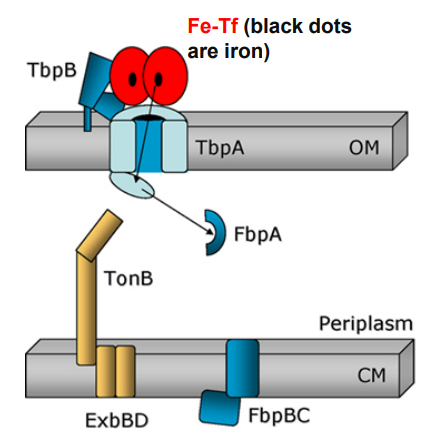
What is the first step of Neisseria using TonB-dependent Outer Membrane transporters to move Ferrated transferrin (Fe-Tf)?
Ferrated transferrin (Fe-Tf) binds to TbpA and TbpB
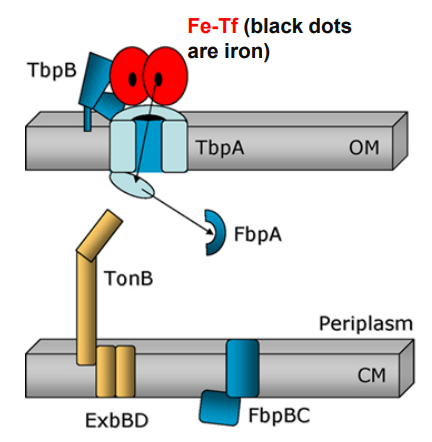
What happens when Neisseria uses TonB-dependent Outer Membrane transporters after this step?
1. Ferrated transferrin (Fe-Tf) binds to TbpA and TbpB
-TbpA and TbpB extract iron from Fe-Tf
-Free iron is captured by a TbpA domain called the plug domain
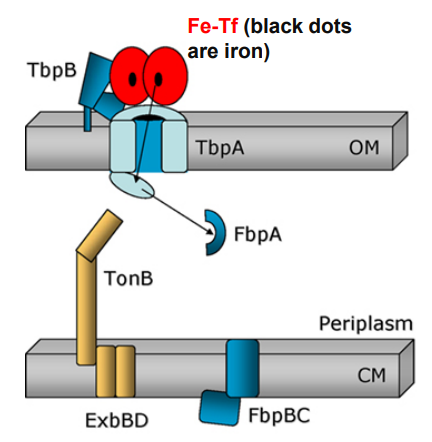
What happens when Neisseria uses TonB-dependent Outer Membrane transporters after this step?
2. TbpA and TbpB extract iron from Fe-Tf and iron is captured by TbpA plug domain
-Exposure of the TonB binding domain of TbpA
-Signals to TonB that iron is bound and ready for transport.
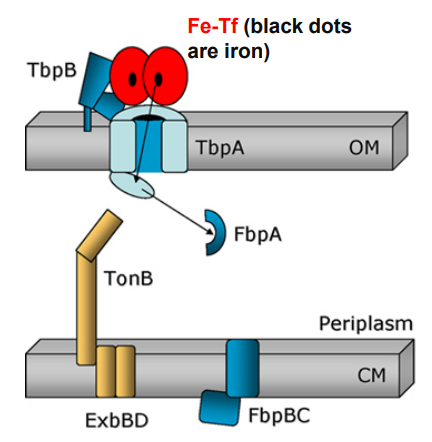
What happens when Neisseria uses TonB-dependent Outer Membrane transporters after this step?
3. Exposure of the TonB binding domain of TbpA
-TonB in its energised form (assisted by ExbBD) tugs on the plug domain
-Rearrangement/presentation of iron atom at the end of the TbpA barrel
-TonB pulls the plug
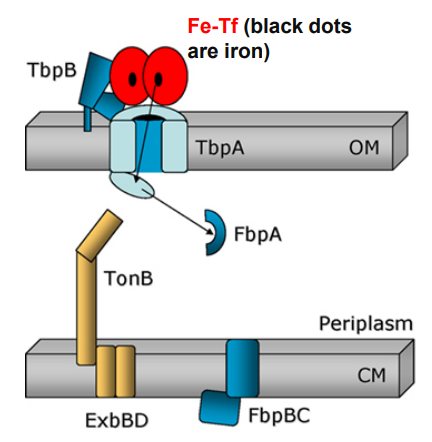
What happens when Neisseria uses TonB-dependent Outer Membrane transporters after this step?
-TonB in its energized form (assisted by ExbBD) tugs on the plug domain
Plug is denatured and iron is released into periplasm
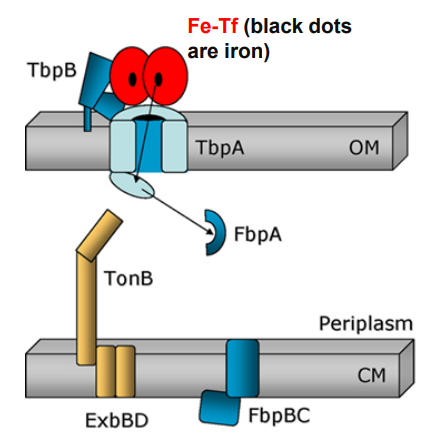
What happens when Neisseria uses TonB-dependent Outer Membrane transporters after this step?
-Plug domain is denatured and iron is released into periplasm
FbpA captures free iron for transport across the periplasm
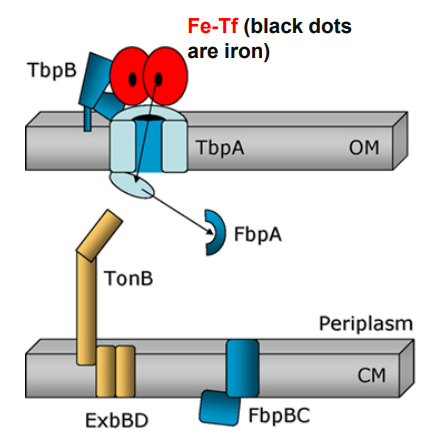
What happens when Neisseria uses TonB-dependent Outer Membrane transporters after this step?
-Free iron is captured by FbpA for transport across the periplasm
TbpB facilitates apo-Tf release from surface
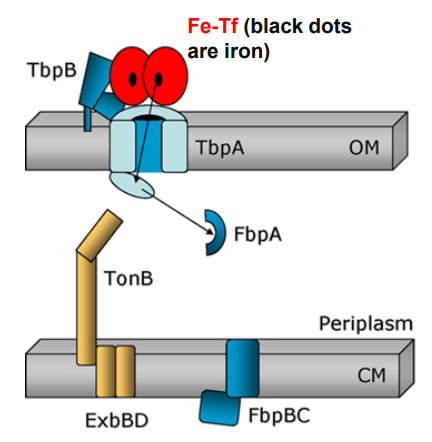
What happens when Neisseria uses TonB-dependent Outer Membrane transporters after this step?
-TbpB facilitates apo-Tf release from surface
-FbpA associates with FbpB permease in the cytoplasmic membrane
-FbpC generates ATP
-Iron crosses cytoplasmic membrane and enters the cytoplasm
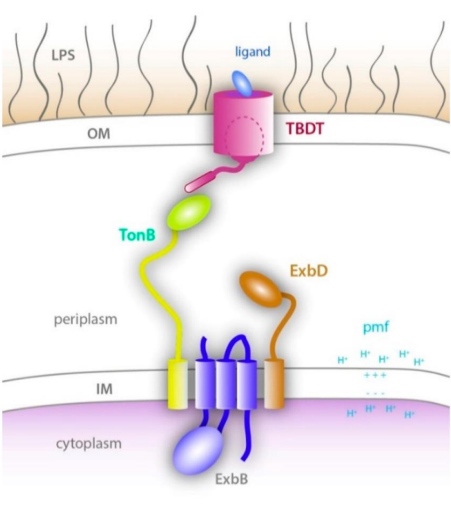
How does the Ton complex interact with the TonB Dependent Transporter (TBDT) in the Outer Membrane?
1) Ligand binds to TBDT
2) TonB interacts with TBDT box
3) PMF energy produced by Ton complex
4) Ejects TBDT plug domain
5) Ligand imported across Outer Membrane
Which iron-capturing mechanism is this?
-Pathogen lyses erythrocytes
-Digests the hemoglobin
-Assimilates heme which contains iron
Hemolysins
What is this?
-Bacterial virulence is associated with Pneumolysin
Streptococcus pneumoniae
What is this?
-Binds Cholesterol in host cell membranes
-Disrupts them by forming pores
-Found in Strep pneumoniae
Pneumolysin
What is this?
-Bacteria virulence is associated with Alpha hemolysin
Systemic E. coli
What is this?
-Hemolysin produced by Systemic E. coli
-Not produced by non-invasive fecal E. coli
Alpha hemolysin
True or False: In uropathogenic E. coli, hemolysin is linked to increased disease severity and hemorrhagic infection
True
How does a pathogen take up heme from the host environment?
-Gram Negative bacteria secrete hemophores
-Bind to heme and bring it to a cell surface receptor
-Directly bind to heme
How do hemophore proteins collect heme?
-Bind heme and bring it to a receptor on the cell surface
-Can also directly bind to heme
True or False: Heme transport across the outer and inner membranes does not depend on TonB
False
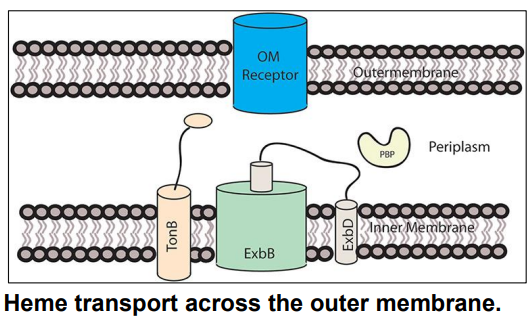
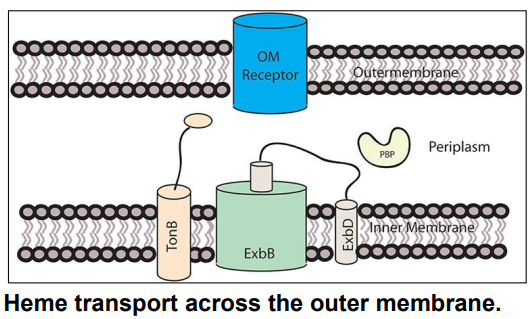
What is this?
-Transports heme across OM
-Uses PMF to transfer energy for transport to OM receptor
TonB-ExbB-ExbD
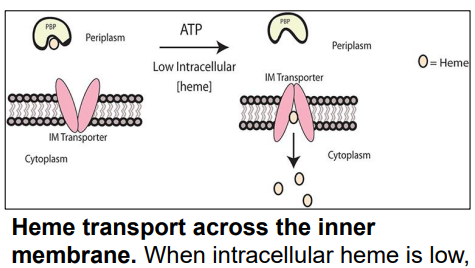
What is this?
-Transports heme across Inner Membrane
-Transfers heme from periplasm → cytoplasm via conformational change
ABC Transporter
What is this?
-Enzyme that removes iron from heme after transport
Heme oxygenase
What is the difference between the 3 methods of iron acquisition?
1) Hemolysins release iron from RBC
2) Siderophores chelate iron from host iron-containing proteins and transfer it to bacterial cells
3) Direct contact bacteria bind iron-containing host proteins and extract the iron
What type of transport is TonB dependent?
Transport across the Gram Negative Outer Membrane
How is iron sequestered in the host to stop bacterial growth? (slide 4)
-Iron-binding proteins will sequester/tightly bind iron
-Transferrin: Serum protein in the liver responsible for iron transport in blood and tissue
-Lactoferrin: Secreted at mucosal surfaces, found in milk, saliva, tears
-Ferritin: Used for intracellular iron storage
-Hemoglobin: Contains heme and 70% of total body iron in RBCs
What are siderophores? How do they work to collect iron? (slide 5- 7)
1) Low molecular weight compounds that chelate iron with very high affinity, secreted into medium/tissues
2) Forms complex with Fe3+, removing it from host protein
3) Iron-Siderophore complex enters bacterial cell via specific transport mechanism
4) Ferric reductase reduces Fe3+ to Fe2+ and releases it
Note: Siderophore synthesis is inhibited by Fur repressor which binds Fe2+ w/ high affinity
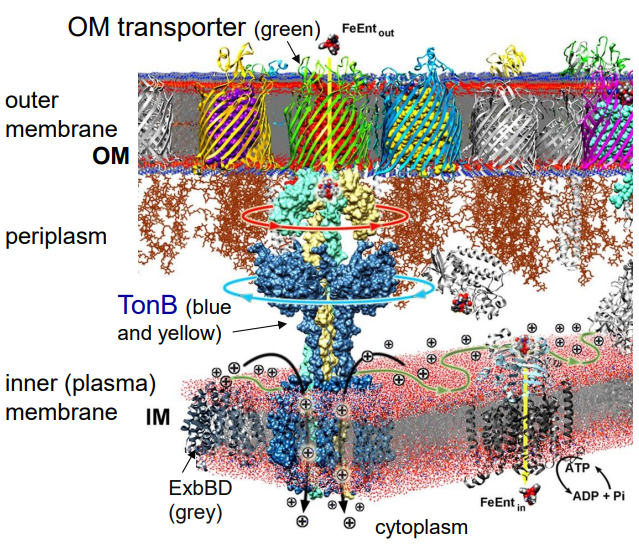
Using ferric enterobactin (FeEnt) as your example, explain how siderophores are transported into the bacterial cell without ATP (slide 7)
1) TonB rotation powered by PMF
2) Promotes conformational change of Outer Membrane Transporter
3) FeEnt passes through to Inner Membrane
4) ABC transporter uses ATP to transport FeEnt into cell
Why do bacteria produce more than one siderophore? (slide 9)
-Siderophores do different jobs in different environments or at different times during infection
-Trading off efficiency versus cost of siderophore production
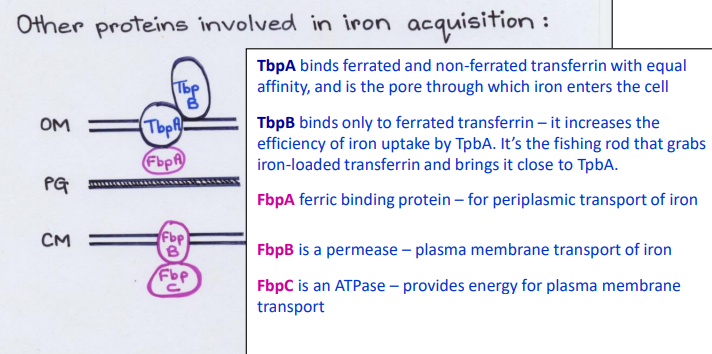
How do bacteria steal iron from transferrin? (slide 12)
1) Bacteria bind directly to host transferrin or lactoferrin
2) TbpA pore channel binds ferrated and non-ferrated transferrin with equal affinity in the Outer Membrane
3) TbpB carrier binds only ferrated transferrin and brings it close to TbpA
4) FbpA carrier is in the periplasm
5) FbpB channel is a permease in plasma membrane
6) FbpC is an ATPase for FbpB channel
How does TonB assist OM-transporters to import iron from transferrin? (slide 14)
1) Ferrated transferrin (Fe-Tf) binds to TbpA and TbpB
2) TbpA and TbpB extract iron from Fe-Tf
3) TbpA plug domain captures iron
4) TbpA exposes its TonB-binding domain
4) TonB (assisted by ExbBD) tugs on Tbpa plug domain
5) TbpA plug domain is denatured, releases free iron into periplasm
6) FbpA captures free iron for transport across the periplasm (while TbpB facilitates apo-Tf release from surface)
7) FbpA associates with FbpB channel in plasma membrane
8) FbpC ATPase provides energy to move iron into the cytoplasm
How do bacteria obtain iron from heme? (slide 16-17)
1) Gram Negative bacteria secrete proteins called hemophores that directly bind/bring heme to cell surface receptor
2) TonB-ExbB-ExbD in the inner membrane uses PMF to transport heme to across Outer Membrane
3) ABC transporter in the inner membrane uses ATP to transfer heme from periplasm → cytoplasm via a conformational change
4) Heme oxygenase removes iron from heme