2_Microscopic Visualization of Bacteria
1/84
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
85 Terms
Mycobacterium tuberculosis
the bacterium that causes tuberculosis, can be detected in specimens based on the presence of acid-fast bacilli
acid-fast staining (Ziehl-Neelsen) technique
is being used for diagnosis of tuberculosis in which M. tuberculosis is detected in smears prepared from a sample of a patient’s sputum.
Prepare smears of and Mycobacterium smegmatis and Staphylococcus aureus on clean glass slides, following the procedures in smear preparation.
Cover each smear with a piece of filter paper that has been cut to the same size as the bacterial smear.
Within a fume hood with exhaust fan on, place the slide on a hot plate or steaming water bath and saturate the paper with Ziehl’s carbol fuchsin.
Heat for 3 to 5 minutes. You may add few drops of the stain to prevent slides from drying out and also, adjust the temperature of the hot plate to prevent boiling.
Remove the slide, let it cool, and rinse with water for 30 seconds while the slide rests on the staining rack.
Add acid-alcohol drop by drop until the slide gets decolorized into slightly pink. This must be done carefully within 10 to 30 seconds.
Rinse with water for 5 seconds while the slide rests on the staining rack.
Counterstain with alkaline methylene blue for about 2 minutes.
Rinse with water for 30 seconds while the slide rests on the staining rack.
Blot dry with bibulous paper.
Examine slide on a microscope under oil-immersion objective (100X).
E. coli
is a gram-negative non-sporeforming rod
S. faecalis
is a gram-positive coccus.
gram-positive coccus, enterococcus
S. faecalis is classified as an
CV. GI. D. S.
1-1-15s-30/45s
Gram staining procedure
alcohol treatment
The amount of ___ (the differential stage) must be judged carefully because over-treatment washes the crystal violet- iodine complex from Gram-positive bacteria and they will appear to be Gram-negative.
differential stage
The amount of alcohol treatment (the___) must be judged carefully because over-treatment washes the crystal violet- iodine complex from Gram-positive bacteria and they will appear to be Gram-negative.
lose the ability to retain the crystal violet-iodine complex
Always use a young culture because older cultures of Gram-positive bacteria tend to ___ and appear to be Gram-negative; but some bacteria are naturally only weakly Gram-positive.
Purple
Gram-positive bacteria will stain
Red to Pink
Gram-negative bacteria will stain
Ziehl’s carbol fuchsin
In Acid-fast (Ziehl-Neelsen) Technique:
Prepare smears of and Mycobacterium smegmatis and Staphylococcus aureus on clean glass slides, following the procedures in smear preparation.
Cover each smear with a piece of filter paper that has been cut to the same size as the bacterial smear.
Within a fume hood with exhaust fan on, place the slide on a hot plate or steaming water bath and saturate the paper with ___
Heat for 3 to 5 minutes. You may add few drops of the stain to prevent slides from drying out and also, adjust the temperature of the hot plate to prevent boiling.
Remove the slide, let it cool, and rinse with water for 30 seconds while the slide rests on the staining rack.
Add acid-alcohol drop by drop until the slide gets decolorized into slightly pink. This must be done carefully within 10 to 30 seconds.
Rinse with water for 5 seconds while the slide rests on the staining rack.
Counterstain with alkaline methylene blue for about 2 minutes.
Rinse with water for 30 seconds while the slide rests on the staining rack.
Blot dry with bibulous paper.
Examine slide on a microscope under oil-immersion objective (100X).
acid-alcohol
In Acid-fast (Ziehl-Neelsen) Technique:
Prepare smears of and Mycobacterium smegmatis and Staphylococcus aureus on clean glass slides, following the procedures in smear preparation.
Cover each smear with a piece of filter paper that has been cut to the same size as the bacterial smear.
Within a fume hood with exhaust fan on, place the slide on a hot plate or steaming water bath and saturate the paper with Ziehl’s carbol fuchsin.
Heat for 3 to 5 minutes. You may add few drops of the stain to prevent slides from drying out and also, adjust the temperature of the hot plate to prevent boiling.
Remove the slide, let it cool, and rinse with water for 30 seconds while the slide rests on the staining rack.
Add ___ drop by drop until the slide gets decolorized into slightly pink. This must be done carefully within 10 to 30 seconds.
Rinse with water for 5 seconds while the slide rests on the staining rack.
Counterstain with alkaline methylene blue for about 2 minutes.
Rinse with water for 30 seconds while the slide rests on the staining rack.
Blot dry with bibulous paper.
Examine slide on a microscope under oil-immersion objective (100X).
slightly pink
In Acid-fast (Ziehl-Neelsen) Technique:
Prepare smears of and Mycobacterium smegmatis and Staphylococcus aureus on clean glass slides, following the procedures in smear preparation.
Cover each smear with a piece of filter paper that has been cut to the same size as the bacterial smear.
Within a fume hood with exhaust fan on, place the slide on a hot plate or steaming water bath and saturate the paper with Ziehl’s carbol fuchsin.
Heat for 3 to 5 minutes. You may add few drops of the stain to prevent slides from drying out and also, adjust the temperature of the hot plate to prevent boiling.
Remove the slide, let it cool, and rinse with water for 30 seconds while the slide rests on the staining rack.
Add acid-alcohol drop by drop until the slide gets decolorized into __. This must be done carefully within 10 to 30 seconds.
Rinse with water for 5 seconds while the slide rests on the staining rack.
Counterstain with alkaline methylene blue for about 2 minutes.
Rinse with water for 30 seconds while the slide rests on the staining rack.
Blot dry with bibulous paper.
Examine slide on a microscope under oil-immersion objective (100X).
alkaline methylene blue
In Acid-fast (Ziehl-Neelsen) Technique:
Prepare smears of and Mycobacterium smegmatis and Staphylococcus aureus on clean glass slides, following the procedures in smear preparation.
Cover each smear with a piece of filter paper that has been cut to the same size as the bacterial smear.
Within a fume hood with exhaust fan on, place the slide on a hot plate or steaming water bath and saturate the paper with Ziehl’s carbol fuchsin.
Heat for 3 to 5 minutes. You may add few drops of the stain to prevent slides from drying out and also, adjust the temperature of the hot plate to prevent boiling.
Remove the slide, let it cool, and rinse with water for 30 seconds while the slide rests on the staining rack.
Add acid-alcohol drop by drop until the slide gets decolorized into slightly pink. This must be done carefully within 10 to 30 seconds.
Rinse with water for 5 seconds while the slide rests on the staining rack.
Counterstain with ____for about 2 minutes.
Rinse with water for 30 seconds while the slide rests on the staining rack.
Blot dry with bibulous paper.
Examine slide on a microscope under oil-immersion objective (100X).
alkaline methylene blue
Counter stain for Acid-fast Staining (Ziehl-Neelsen technique)
Purple
M. smegmatis in Acid-fast appears to be
blue
S. aureus in Acid-fast appears to be
red
Acid-fast organisms stain
blue or brown
The background and other organisms in Acid-fast stain
Endospore staining (Schaeffer-Fulton technique)
Prepare smears of a 24-hour and a 7- day old cultures of Bacillus subtilis on clean glass slides, following the procedures in smear preparation.
Cover the smear with a piece of filter paper that has been cut to the same size as the bacterial smear.
Within a fume hood with exhaust fan on, put the slide on top of beaker with boiling water, placed on a hot plate and saturate the paper with Malachite Green solution.
Steam slide for 5 minutes. You may add few drops of the stain to prevent slides from drying out and also, adjust the temperature of the hot plate to prevent overheating.
Remove slide from beaker, remove and discard the filter paper, allow slide to cool for 2 minutes.
Holding slide at an angle, rinse thoroughly by squirting a gentle, indirect stream of water onto slide, allowing it to drain down over smear.
Holding slide level, flood smear with Safranin, allow to stand for 1 minute.
Rinse excess Safranin as in step 6 above.
Allow to air-dry.
Examine slide on a microscope under oil-immersion objective (100X).
Malachite Green solution
In Endospore staining (Schaeffer-Fulton technique):
Prepare smears of a 24-hour and a 7- day old cultures of Bacillus subtilis on clean glass slides, following the procedures in smear preparation.
Cover the smear with a piece of filter paper that has been cut to the same size as the bacterial smear.
Within a fume hood with exhaust fan on, put the slide on top of beaker with boiling water, placed on a hot plate and saturate the paper with ___.
Steam slide for 5 minutes. You may add few drops of the stain to prevent slides from drying out and also, adjust the temperature of the hot plate to prevent overheating.
Remove slide from beaker, remove and discard the filter paper, allow slide to cool for 2 minutes.
Holding slide at an angle, rinse thoroughly by squirting a gentle, indirect stream of water onto slide, allowing it to drain down over smear.
Holding slide level, flood smear with Safranin, allow to stand for 1 minute.
Rinse excess Safranin as in step 6 above.
Allow to air-dry.
Examine slide on a microscope under oil-immersion objective (100X).
Safranin
Because gram negative bacteria are colorless after the alcohol wash, the addition of ___ (the counterstain) turns the cells pink or red.
provides a contrasting color to the primary stain (crystal violet).
Counterstain in Endospore staining
is applied to the smear to stain portions of the cell other than endospores.
In Endospore staining (Schaeffer-Fulton technique):
Prepare smears of a 24-hour and a 7- day old cultures of Bacillus subtilis on clean glass slides, following the procedures in smear preparation.
Cover the smear with a piece of filter paper that has been cut to the same size as the bacterial smear.
Within a fume hood with exhaust fan on, put the slide on top of beaker with boiling water, placed on a hot plate and saturate the paper with Malachite Green solution.
Steam slide for 5 minutes. You may add few drops of the stain to prevent slides from drying out and also, adjust the temperature of the hot plate to prevent overheating.
Remove slide from beaker, remove and discard the filter paper, allow slide to cool for 2 minutes.
Holding slide at an angle, rinse thoroughly by squirting a gentle, indirect stream of water onto slide, allowing it to drain down over smear.
Holding slide level, flood smear with __, allow to stand for 1 minute.
Rinse excess Safranin as in step 6 above.
Allow to air-dry.
Examine slide on a microscope under oil-immersion objective (100X).
green
In Endospore staining, Spores will stain
red
In Endospore staining, Vegetative cells will stain
Capsular Staining
Aseptically transfer two loopfuls of Klebsiella pneumoniae on one side of the slide and mix it well with a small drop (or 10 µL) of 1% Congo Red solution. Prepare another slide for Proteus vulgaris.
Use another clean slide (spreader) and touch one edge to the mixture, allowing the mixture to run across the slide’s width.
Hold the spreader at about 30⁰ angle and push it swiftly to spread the mixture toward the opposite end of the first slide to produce a thin even layer.
Allow the newly formed smear to completely air-dry for 5-7 minutes. DO NOT heat-fix as heating can dehydrate or distort the capsule.
Cover the smear with 1% Crystal Violet for 1 minute.
Gently wash off excess crystal violet by holding the slide at an angle and apply a gentle stream of water onto the slide for 5 seconds. The water should run down indirectly over the stained bacteria.
Hold the slide at a 45⁰ angle until completely air-dried.
Examine slide on a microscope under oil-immersion objective (100X).
1% Congo Red solution
In Capsular staining:
Aseptically transfer two loopfuls of Klebsiella pneumoniae on one side of the slide and mix it well with a small drop (or 10 µL) of ___ . Prepare another slide for Proteus vulgaris.
Use another clean slide (spreader) and touch one edge to the mixture, allowing the mixture to run across the slide’s width.
Hold the spreader at about 30⁰ angle and push it swiftly to spread the mixture toward the opposite end of the first slide to produce a thin even layer.
Allow the newly formed smear to completely air-dry for 5-7 minutes. DO NOT heat-fix as heating can dehydrate or distort the capsule.
Cover the smear with 1% Crystal Violet for 1 minute.
Gently wash off excess crystal violet by holding the slide at an angle and apply a gentle stream of water onto the slide for 5 seconds. The water should run down indirectly over the stained bacteria.
Hold the slide at a 45⁰ angle until completely air-dried.
Examine slide on a microscope under oil-immersion objective (100X).
dehydrate or distort the capsule.
In Capsular staining:
Aseptically transfer two loopfuls of Klebsiella pneumoniae on one side of the slide and mix it well with a small drop (or 10 µL) of 1% Congo Red solution. Prepare another slide for Proteus vulgaris.
Use another clean slide (spreader) and touch one edge to the mixture, allowing the mixture to run across the slide’s width.
Hold the spreader at about 30⁰ angle and push it swiftly to spread the mixture toward the opposite end of the first slide to produce a thin even layer.
Allow the newly formed smear to completely air-dry for 5-7 minutes. DO NOT heat-fix as heating can ___
Cover the smear with 1% Crystal Violet for 1 minute.
Gently wash off excess crystal violet by holding the slide at an angle and apply a gentle stream of water onto the slide for 5 seconds. The water should run down indirectly over the stained bacteria.
Hold the slide at a 45⁰ angle until completely air-dried.
Examine slide on a microscope under oil-immersion objective (100X).
1% Crystal Violet
In Capsular staining:
Aseptically transfer two loopfuls of Klebsiella pneumoniae on one side of the slide and mix it well with a small drop (or 10 µL) of 1% Congo Red solution. Prepare another slide for Proteus vulgaris.
Use another clean slide (spreader) and touch one edge to the mixture, allowing the mixture to run across the slide’s width.
Hold the spreader at about 30⁰ angle and push it swiftly to spread the mixture toward the opposite end of the first slide to produce a thin even layer.
Allow the newly formed smear to completely air-dry for 5-7 minutes. DO NOT heat-fix as heating can dehydrate or distort the capsule.
Cover the smear with ___for 1 minute.
Gently wash off excess crystal violet by holding the slide at an angle and apply a gentle stream of water onto the slide for 5 seconds. The water should run down indirectly over the stained bacteria.
Hold the slide at a 45⁰ angle until completely air-dried.
Examine slide on a microscope under oil-immersion objective (100X).
purple
In capsular staining, Bacterial cells will stain
dark
In capsular staining,Background of the slide will stain
clear halo
In capsular staining, Capsules will be ___ around cells against a dark background.
Crystal violet
In gram staining, ___, the primary stain, stains both gram positive and gram-negative cells purple because the dye enters the cytoplasm of both types of cells.
cytoplasm
In gram staining, Crystal violet, the primary stain, stains both gram positive and gram-negative cells purple because the dye enters the __ of both types of cells.
iodine
In gram staining, When __(the mordant) is applied, it forms large crystals with the dye that are too large to escape through the cell wall.
large crystals
In gram staining, When iodine (the mordant) is applied, it forms __ with the dye that are too large to escape through the cell wall.
alcohol
In gram staining, The application of __ dehydrates the peptidoglycan of gram-positive cells to make it more impermeable to the crystal violet-iodine.
dehydrates
In gram staining, The application of alcohol ___the peptidoglycan of gram-positive cells to make it more impermeable to the crystal violet-iodine.
impermeable
In gram staining, The application of alcohol dehydrates the peptidoglycan of gram-positive cells to make it more ___ to the crystal violet-iodine.
small holes
The effect on gram-negative cells is quite different; alcohol dissolves the outer membrane of gram negative cells and even leaves ___in the thin peptidoglycan layer through which crystal violet-iodine diffuse.
Bacillus and Clostridium
are examples and are often described as gram-variable.
acid-fast stain
The ___ is used to identify all bacteria of the genus Mycobacterium and pathogenic species of Nocardia
Mycobacterium and Nocardia
The acid-fast stain is used to identify all bacteria of the genus ___and pathogenic species of ___
These bacteria contain high concentrations (60%) of a hydrophobic waxy lipid (mycolic acid) in their cell wall that prevents the uptake of dyes, including those used in the Gram stain.
carbolfuchsin
Acid-fast bacteria can be stained with___ , which penetrates bacteria more effectively when heated.
penetrates the cell wall, binds to the cytoplasm, and resists removal by washing with acid-alcohol.
endospore
An ___ is a special resistant, dormant structure formed within a cell that protects a bacterium from adverse environmental conditions.
C. botulinum
causes botulism which is obtained from food, usually canned or bottled food.
Malachite green
Primary stain in Endospore staining
Schaeffer-Fulton endospore stain
The most commonly used stain in endospore stain is the
heat
In endospore staining, The __ helps the stain penetrate the endospore wall.
Safranin
Counterstain in Endospore staining
is applied to the smear to stain portions of the cell other than endospores.
green within red or pink cells
In a properly prepared smear, the endospores appear ___
capsule
Some bacteria have a layer of material lying outside the cell wall. When the layer is well organized and not easily washed off, it is called a __
slime layer
A ___ is a zone of diffuse, unorganized material that is removed easily.
glycocalyx
A ___is a network of polysaccharides extending from the surface of bacteria and other cells (in this sense it could encompass both capsules and slime layers).
polysaccharides
Capsules and slime layers usually are composed of ___, but they may be constructed of other materials.
Bacillus anthracis
has a capsule of poly-D-glutamic acid
poly-D-glutamic acid
Bacillus anthracis has a capsule of
water soluble and may be dislodged or removed
Capsule staining is more difficult than other types of staining procedures because capsular materials are ____ during rigorous washing.
India ink, Congo red solution or Nigrosin
To demonstrate the presence of capsules, the bacteria is mixed in a solution containing a fine colloidal suspension of colored particles (usually___) to provide a contrasting background and then the bacteria is stained with a simple stain, such as safranin.
resist phagocytosis by host phagocytic cells
Although capsules are not required for bacterial growth and reproduction in laboratory cultures, they do confer several advantages when bacteria grow in their normal habitats. They help bacteria ____
destroyed easily and does not cause disease
When a bacteria lacks a capsule, it is___
Bacillus anthracis (anthrax), Clostridium tetani (tetanus), C. botulinum (botulism) and C. perfringens (gas gangrene)
are medically important bacteria, known to produce endospores (spore-formers).
Escherichia coli and Streptococcus faecalis
are considered indicator organisms of fecal contamination in assessing water quality and sewage status.
Their presence in samples make them good indicators because they are normally not present in water or soil, they are relatively easy to identify, and they survive a little longer in water than enteric pathogens.
The two are completely different organisms.
Simple, Differentia, Special
3 types of staining:
Simple Staining
What type of staining:
1. use of a single dye and reveals basic cell shapes and cell arrangements.
2. Methylene blue, safranin, carbol fuchsin, and crystal violet are commonly used
Differential staining
What type of staining:
makes use of two or more dyes and distinguishes between two kinds of organisms
Example:- Gram Stain, Acid Fast staining
Special staining
What type of staining:
to study specific bacterial structures with the light microscope.
Example:
1. Negative staining - capsule
2. Schaeffer-Fulton spore stain
3. Flagellar stain- Flagella appear as dark lines with silver, or red with carbol fuchsin
Basic Dye

Acidic Dye
mycobacterial outer membrane, middle layer or the periplasmic cell wall, cytoplasmic phospholipid bilayer
The Mycobacterium Envelope is subdivided into three layers:
mycobacterial outer membrane
Which layer of the Mycobacterium Envelope?
comprised of mycolic acids and various lipids such as branched and capped portions of lipoarabinomannans.
middle layer or the periplasmic cell wall
Which layer of the Mycobacterium Envelope?
consists mainly of peptidoglycans and galactans, some lipomannan portions of lipoarabinomannans; significant amounts of phosphatidylinositol mannosides that link the periplasmic cell wall to the inner third layer
cytoplasmic phospholipid bilayer
Which layer of the Mycobacterium Envelope?
This layer consists mainly of basic plasma membrane phospholipids as well as some polyprenyl sugars.
“Some Killers Have Pretty Nice Capsule”
(Streptococcus pneumoniae, Klebsiella pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa, Neisseria meningitidis, Cryptococcus neoformans)
Encapsulated bacteria:
Smear preparation • Fixation • Staining
3 BASIC STEPS IN STAINING MICROORGANISM
smear
a thin dry film of microorganisms
chromophoric ions
A dye is an organic compound carrying
Enhances contrast between bacteria and the surrounding material
Permits observation in great detail, and better resolution in microscopy
Purpose of Dyes
absorption, adsorption, osmosis, capillary action, ion-exchange
Mechanisms of Staining
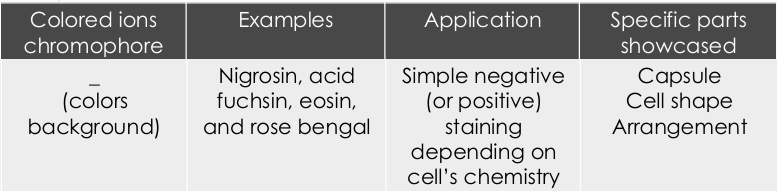
Positive staining
In ____- a dye is absorbed by the cells or organisms being observed, adding color to objects of interest to make them stand out against the background.
Negative staining
In ____ - dyes is repelled by the negatively charged cell parts and is absorbed by the background.
stain absorption
Simple positive staining – intends to show cell structures by ___
stain repulsion
Simple negative staining - intends to show cell structures by ___ (only the background is colored)