Metallurgy
1/41
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
Minerals
Metallic compounds that occur naturally on the Earth's crust.
Ores
Minerals from which metals can be extracted easily and profitably
Limestone
CaCO3
Bauxite
Al2O3.2H2O
Cryolite
Na3AlF6
Corundum
Al2O3
Red haematite
Fe2O3
Brown haematite
Fe2O3.3H2O
Magnetite
Fe3O4
Iron Pyrites
FeS2
Siderite
FeCO3
Zinc blende
ZnS
Zincite
ZnO
Calamine
ZnCO3
Fluorspar
CaF2
Alumina
Al2O3
Gangue
Earthly impurities
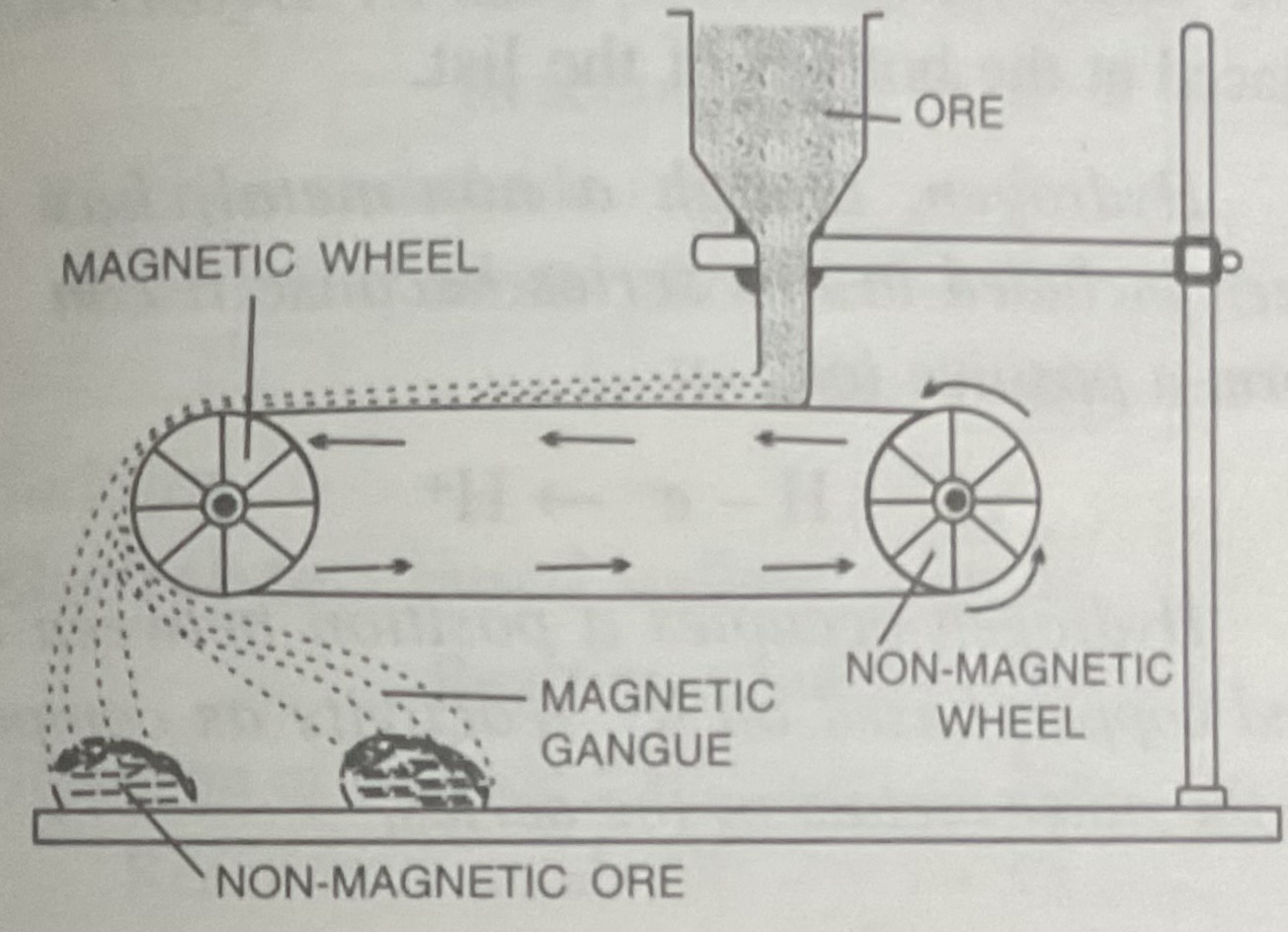
Magnetic seperation
Dressing of iron ores
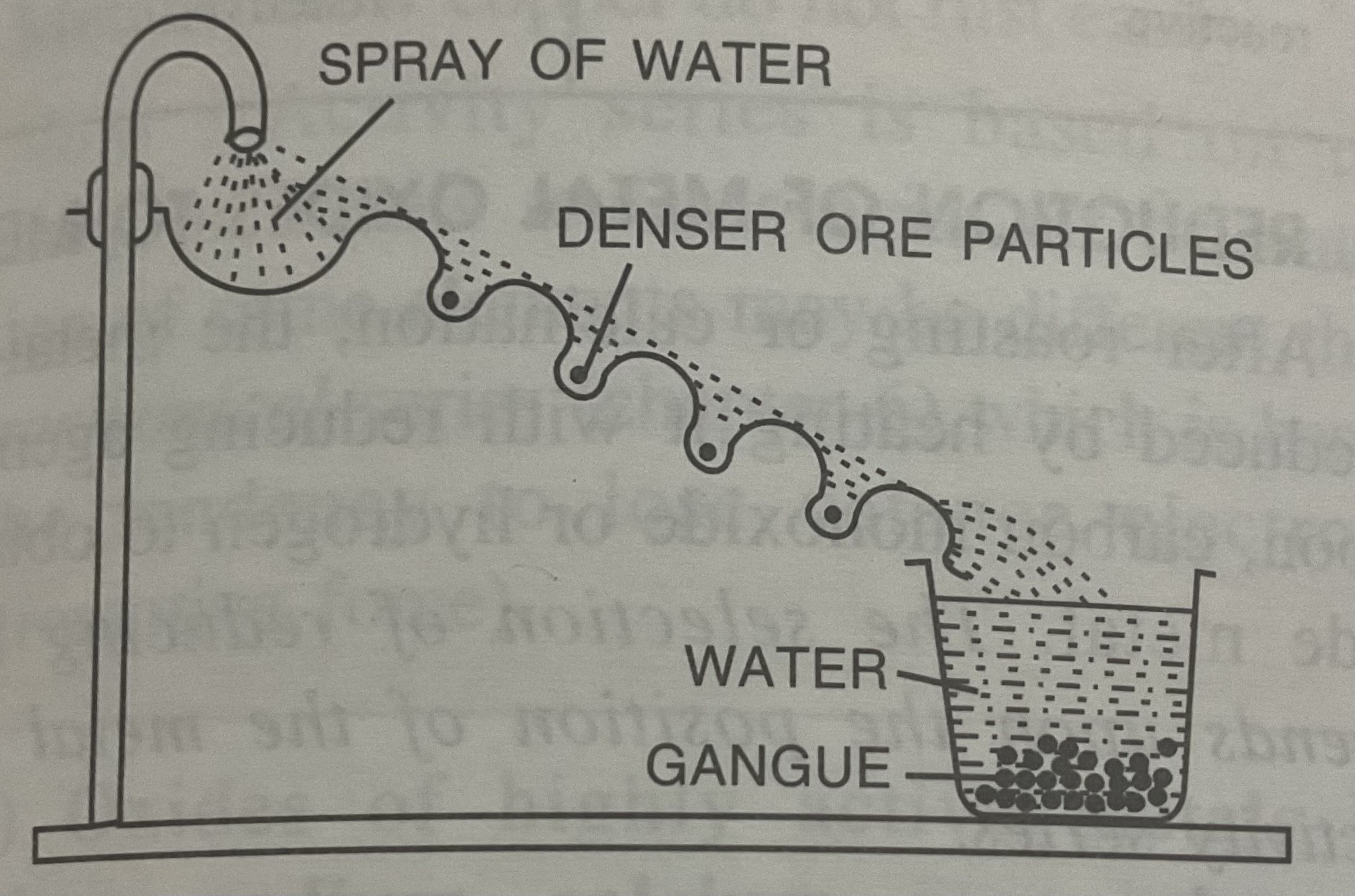
Hydraulic/Hydrolytic washing (or) Gravity separation
Dressing of lead ores
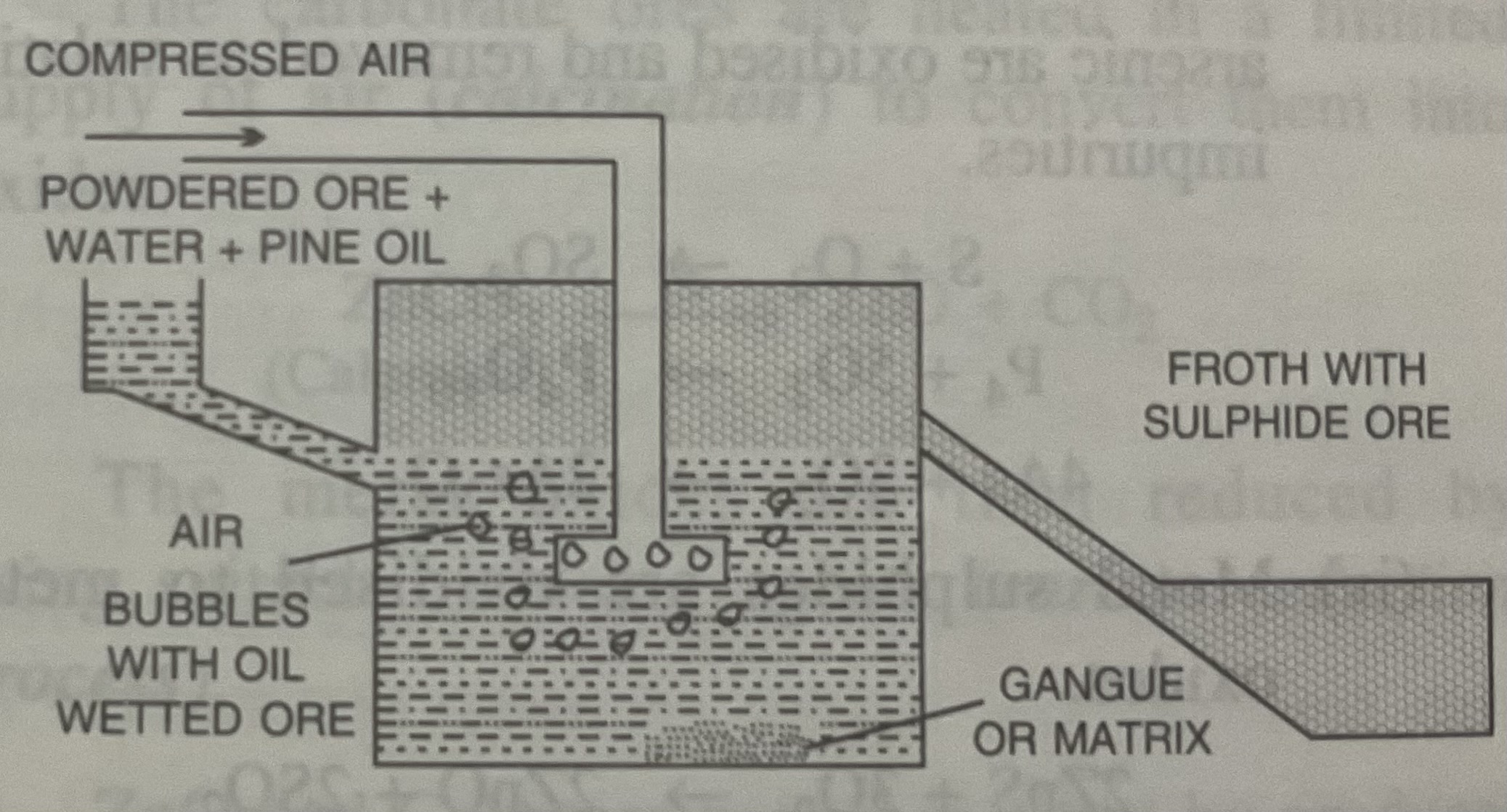
Froth floatation
Dressing of Sulphide ores
Baeyer’s process
Chemical method
Dressing of Aluminium ores
Al2O3.2H2O + NaOH —> 2NaAlO2 + 3H2O
NaAlO2 + H2O —> NaOH + Al(OH)3
Al(OH)3 —> Al2O3 + 3H2O
Stages of extraction
Ore —> Pulverisation —> Dressing/Concentration(Removal of gangue) —> Conversion to oxide(Roasting/Calcination) —> Reduction(Metal Oxide to Metal) —> Purification
Calcination
Heating ore in absence of oxygen
Roasting
Heating ore in presence of oxygen
Reduction of ZnO
chemical method
Reducing agent - Coke(C)
Reduction of Fe2O3
Chemical method
Reducing agent - CO
Reduction of PbO
Chemical method
Reducing agent - NH3
Reduction of CuO
Chemical method
H2
Reduction by electrolyis
K2O, Na2O, CaO, MgO, Al2O3
Reduction by chemical methods
ZnO, CuO, PbO, Fe2O3
Reduction by thermal decomposition
HgO
Hall-Heroult’s process
Electrolytic reduction of alumina to aluminium
Electrolysis is used because bond between aluminium and oxygen is very strong.
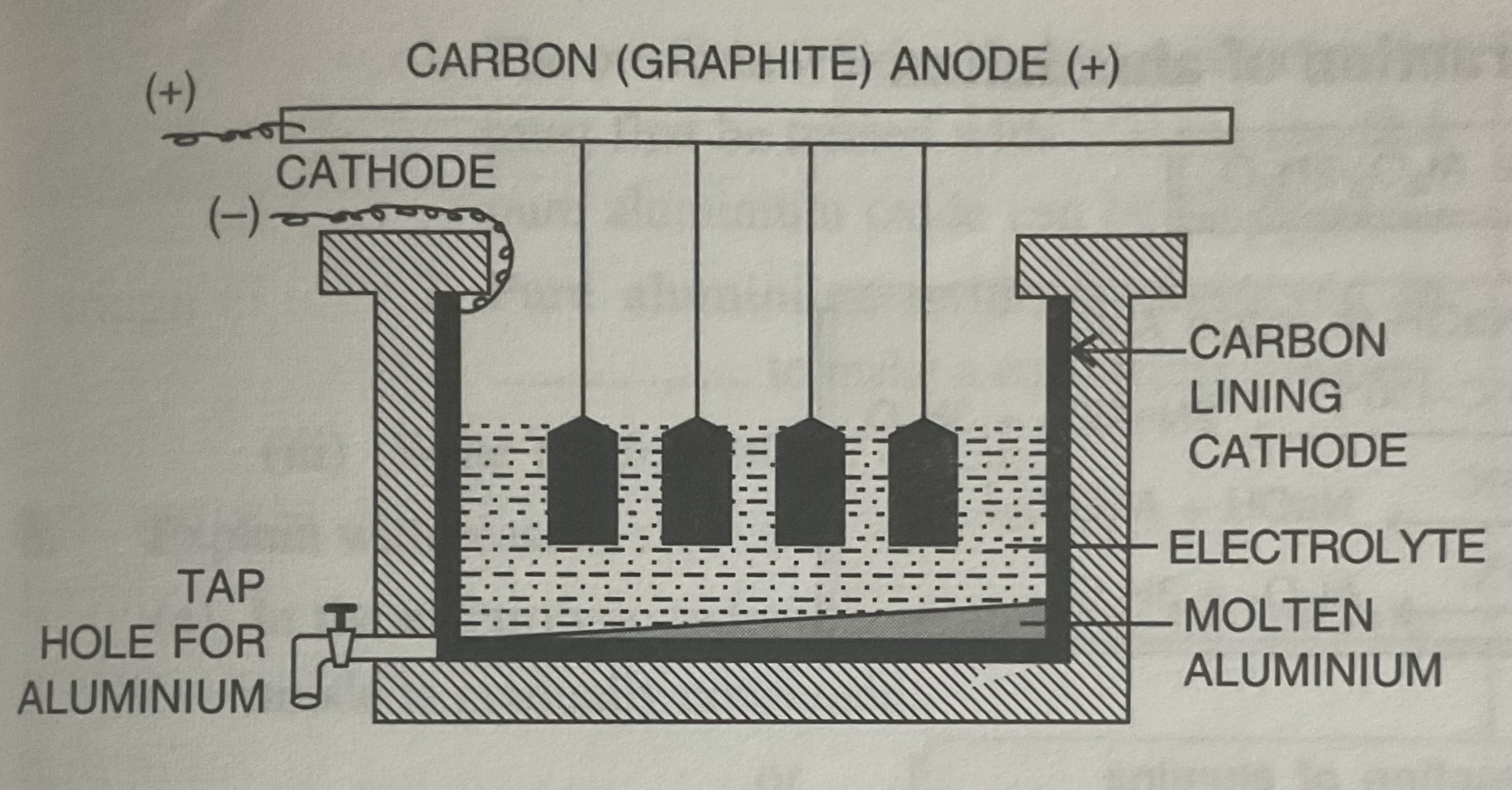
Graphite rods are replaced periodically during Hall-Heroult’s process
Oxygen reacts with graphite and converts it to CO2 OR CO
Why is coke sprinkled over the electrolyte in hall-heroult’s process
Prevents burning of the anode
Reduces heat loss
Role of cryolite and fluorspar in Hall-Heroult’s process
Increases conductivity
Reduces fusion temperature from 2050 to 950
Act as solvent
Alloys
A homogenous mixture of metals in fixed ratios
Duralumin
Al, Cu, Mg, Mn
Making aircrafts
Light tools
Magnalium
Al, Mg
Light tools
Brass
Cu, Zn
Electrical fittings
Decorative articles
Bronze
Statues, medals, coins
Cu, Sn, Zn
Solder
Pb, Sn
Electrical fuse soldering purposes
Stainless steel
Fe, Cr, Ni, C
Utensils, cutlery, surgical instruments