Section 1.5: Energy: A Fundamental Part of Physical and Chemical Changes
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Physical and Chemical Changes are usually accompanied by…
Energy changes. EX: When water evaporates from your skin (physical change) the water molecules absorb energy from your body, making you feel cooler. OR When you burn natural gas on the stove (chemical change) energy is released, heating the food you are cooking.
Energy
the capacity to do work.
Work
defined as the action of a force through a distance. EX: when you push a box across the floor or pedal your bike across the street, you’ve done work.
Kinetic Energy
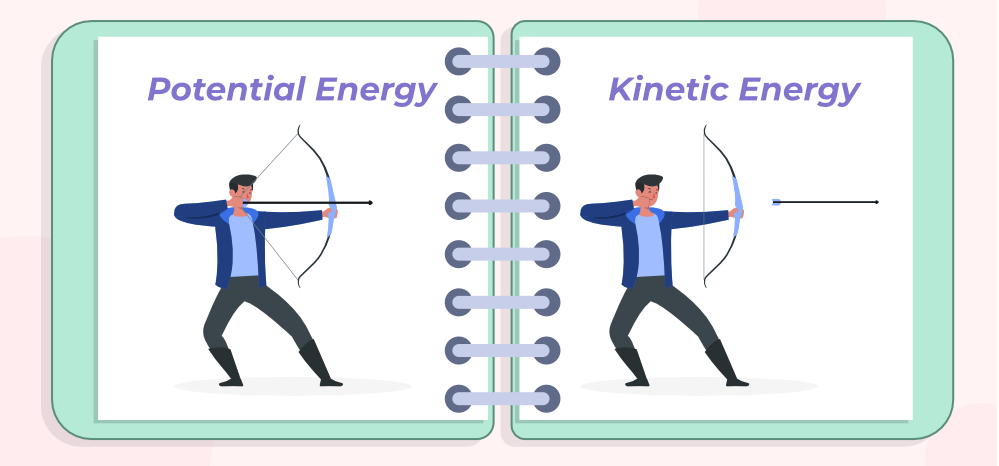
The energy that is transferred is called kinetic energy, which totally depends on the speed and mass of the object in motion. EX: A truck travelling down the road has more kinetic energy than a car travelling at the same speed because the truck’s mass is much more than the car’s. (see image)
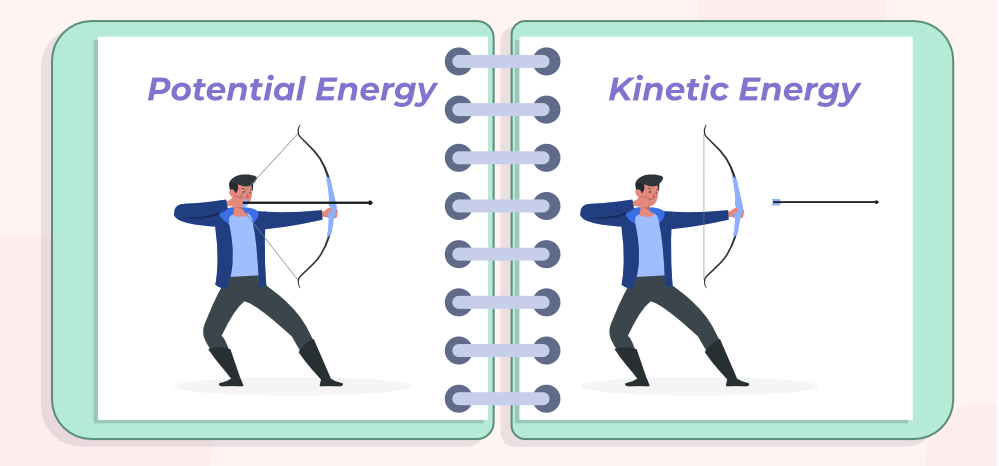
Potential Energy
the energy stored in an object or system due to its position, arrangement, or state. (the energy associated with its position or composition) see image.
Thermal Energy
the internal energy of a substance due to the motion (vibration, rotation, translation) of its atoms and molecules, i.e., the total kinetic energy of its particles, which is why hotter objects have faster-moving particles and colder objects have slower ones. (IN SIMPLE TERMS: the energy from the movement (vibration and motion) of atoms and molecules within a substance).
The total energy of an object is a sum of its…
kinetic energy and its potential energy.
Thermal Energy is a type of…
kinetic energy because it is associated with the motion of the individual atoms or molecules that make up an object. EX: When a weight hits the ground, its kinetic energy is transferred to the atoms and molecules that compose the ground, raising the temperature of the ground ever so slightly.
Energy Conversions
see image

Energy is…
neither created nor destroyed.
Potential energy (of a weight being dropped) becomes…
kinetic energy as the weight accelerates toward the ground.
Kinetic energy (of a weight accelerating towards the ground) becomes…
thermal energy when the weight hits the ground.
The total amount of thermal energy that is released through the process (dropping the weight on the ground) is exactly…
equal to the initial potential energy of the weight.
The law of conservation of energy
Energy is neither created nor destroyed. Energy can change from one type into another, and flow from one object to another, the total quantity of energy does not change, it remains constant.
Objects or systems with high potential energy tend to be…
unstable. EX: the weight lifted several meters from the ground is unstable because it contains a significant amount of potential energy. Unless restrained, the weight will naturally fall, lowering its potential energy (due to its position in Earths gravitational field)
Second Principle of raised weight and its fall…
the tendency of systems with a high potential energy to change in a way that lowers their potential energy. EX: Think of a ball at the top of a hill (high potential energy) rolling down; it converts potential energy to kinetic energy, releasing it until it rests at the bottom (low potential energy). Being that now the weight is at the bottom, and cannot roll off of anything (stable) it now has low potential energy.
Some chemical substances are like a raised weight…
EX: Gasoline is made of molecules that store a lot of energy, kind of like how a raised weight stores energy because it’s high up. These gasoline molecules naturally want to change in a way that lowers their energy. When gasoline burns, that stored energy is released. We can use some of that released energy to do useful things, like making a car move. After the gasoline burns, the new molecules left behind have less energy and are more stable than the gasoline molecules were before.
Gasoline molecules are built in a way that stores a lot of chemical energy (in their bonds).
When gasoline burns, every molecule that reacts goes through the same kind of change.
During combustion, the atoms rearrange into new molecules (like carbon dioxide and water).
Those new molecules have stronger, lower-energy bonds, so they contain less potential energy overall.
SO:
Before burning: gasoline molecules = high energy
After burning: new molecules = lower energy and more stable
Chemical potential energy…
arises primarily from electrostatic forces between the electrically charged particles (protons and electrons) that compose atoms and molecules. Know that molecules contain specific, usually complex, arrangements of these charged particles. Some of these arrangements have a much higher potential energy than others.
Summarizing Energy:
Energy is always conserved in a physical or chemical change; it is neither created nor destroyed.
Systems with high potential energy tend to change in ways that lower their potential energy, transferring energy to the surroundings.
What type of energy is chemical energy?
Kinetic, thermal or potential? ANSWER: Potential energy. Chemical energy is a type of potential energy that results from the electrostatic forces between the charged particles that compose atoms and molecules.