CHEM1602L - Final
1/64
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms
Lab 1: Molecular Weight Determination by Freezing Point Depression
Main purpose/overview?
Determining the molecular weight of an unknown solute by measuring the freezing point of pure water and the freezing point of the solution. From these two values, the delta T freezing was calculated from the equation:
Delta T freezing = Kf*m
Kf for water is 1.86 C/m, and m is the molality of the solute (moles of solute/kg of water). You know the value of m, because you found the weight of the solute as well as the weight of solute + water
Lab 1: Molecular Weight Determination by Freezing Point Depression
Secondary purpose/overview?
Evaluating the Van’t Hoff factor for 0.025 moles of KNOWN ionic compounds (NaCl, CaCl2, K3Fe(CN)6). Since moles of solute and mass of water are known, you know molality. Multiply this molality with Kf for water to get the theoretical delta T. Then, divide observed delta T with theoretical delta T. This is your value for i.
Lab 1: Molecular Weight Determination by Freezing Point Depression
What ions are produced upon dissolving CaCl2?
(Ca)2+ and 2 Cl- ions
Lab 1: Molecular Weight Determination by Freezing Point Depression
What are the ions produced upon dissolving K3Fe(CN)6?
3 K+ ions and (Fe(CN)6)3-
Lab 1: Molecular Weight Determination by Freezing Point Depression
What evidence can you offer that dissolving K3Fe(CN)6 does not yield six aquated cyanide ions (6 CN-)?
The value of i obtained for K3Fe(CN)6 should be much closer to 4 than 10. The observed i value should be around 2.5.
If K3Fe(CN)6 dissolved into 6 CN-, the ideal i would be 10, as K3Fe(CN)6 would dissolve into 6 CN-, 3 K+, and 1 (Fe)3+. However, K3Fe(CN)6 dissolves into 3 K+ and 1 (Fe(CN)6)3-, which has an ideal i value of 4.
Lab 1: Molecular Weight Determination by Freezing Point Depression
What can you conclude about magnitude of charge of anions and the reassociation with cations in the aqueous phase?
The larger the magnitude of charge on the anion, the less stable that anion is when dissolved in water. This is because the larger the magnitude of charge, the more attracted that anion is to cations. Thus, the larger the magnitude of the charge on the anion, the higher the reassociation with cations in the aqueous phase.
Lab 1: Molecular Weight Determination by Freezing Point Depression
Say that you have 8.60 g of an unknown (non-ionizing) solute. You dissolve this solute 50 mL of DI water. The solution reaches a minimum T of -0.7 C. The solution is then measured to have a weight of 67 g. Determine the molecular weight of the unknown solute.
391.3 g/mol
Remember: molality is number of moles of solute divided by kilograms of solvent (not kg of solution)
Lab 1: Molecular Weight Determination by Freezing Point Depression
Say that you dissolve 2.78 g of CaCl2 in 50 mL of DI water. The solution reaches a minimum T of -2.3 C. The mass of the solution is measured to be 59.12 g. What is the real value of i for CaCl2?
i = 2.78
Lab 2: Rate of Reaction
Purpose/overview
To determine the order of reactants in the rate law of the reaction of peroxydisulfate with iodide. Also to determine the rate constant for this reaction, to observe effect of a catalyst, and to determine activation energy
Lab 2: Rate of Reaction
Reaction being studied
(S2O8)2- + 3I- → (I3)- + 2(SO4)2-
Lab 2: Rate of Reaction
Rate Law
rate of decrease of [(S2O8)2-] = k[(S2O8)2-]^m[I-]^n
m and n are determined to both be 1
Lab 2: Rate of Reaction
Why does a yellow color appear in the reaction?
The same amount of (S2O3)2- was added to every mixture. This is because thiosulfate reacts with (I3)- produced by the reaction of (S2O8)2- with I-. Thus, when all of the thiosulfate is consumed, the excess (I3)- creates a yellow color in the solution. Thus, the moles of thiosulfate added is equal to the moles of (S2O8)2- consumed
Lab 2: Rate of Reaction
Calculations for rate
To determine rate, you need to divide 2.5 × 10^-5 moles by the time required for the yellow color to appear. This gives you rate in moles/s
Relative rate is found by dividing all the obtained rates by the slowest rate
Lab 2: Rate of Reaction
Using data without the catalyst, what can you conclude about the effect of [I-] on rate?
As [I-] doubles (and [(S2O8)2-] stays constant), the rate also doubles. Thus, rate is directly proportional to [I-]
Lab 2: Rate of Reaction
What is effect of [(S2O8)2-] on rate?
As [(S2O8)2-] doubles (and [I-] stays constant), the rate also doubles. Thus, rate is also directly proportional to [(S2O8)2-].
Lab 2: Rate of Reaction
A noncatalyzed reaction has [I-] = 0.04 M and [(S2O8)2-] = 0.04 M. This reaction takes 95 seconds for the yellow color to appear. If m = n = 1, what is the value of k for the reaction?
Rate = 2.63 × 10^-7
k = 1.64 × 10^-4 (1/M*s)
Lab 2: Rate of Reaction
Value of k for a catalyzed reaction
Is larger than the value of k for the uncatalyzed reaction
Lab 2: Rate of Reaction
Rates at different temperatures
Rate decreases as temperature decreases
Lab 2: Rate of Reaction
Calculation for activation energy (Ea)
First, calculate the rate of reaction at three different temperatures. Do this by dividing 2.5 × 10^-5 by the time in seconds for the yellow color to appear.
Take the log(relative rates x 100). Then, find 1/T value (in 1/K) for each of the values. Plot a graph where log(rate) is on the y axis, and 1/T is on the x axis. Find the slope of the line of best fit. This slope = -Ea/2.303R (where R = 8.314).
If you plotted ln(k) (or ln(rate)) vs. 1/T, slope = -Ea/R, where R is still 8.314
Lab 3: Chemical Reactions of Copper
Purpose/overview?
To observe sequence of reactions beginning and ending with elemental Cu.
Lab 3: Chemical Reactions of Copper
What happens when nitric acid is added to elemental copper? Write the reaction and state what type of reaction it is.
Cu(s) + 2(NO3)-(aq) + 4 H+(aq) → (Cu)2+(aq) + 2NO2(g) + 2 H2O(l)
Redox reaction
Lab 3: Chemical Reactions of Copper
What happens when concentrated NaOH is added to copper (II) ions? Write the reaction and state what reaction type it is.
(Cu)2+(aq) + 2OH-(aq) → Cu(OH)2(s)
Precipitation reaction
Lab 3: Chemical Reactions of Copper
What happens when you heat copper (II) hydroxide? Write the reaction and state what reaction type it is.
Cu(OH)2(s) → CuO(s) + H2O(g)
Decomposition reaction
The blue Cu(OH)2 decomposes into black/brown powder of copper (II) oxide
Lab 3: Chemical Reactions of Copper
What happens when you add sulfuric acid to copper (II) oxide? Write the reaction and state what reaction type it is
CuO(s) + 2H+(aq) + (SO4)2-(aq) →CuSO4(aq) + H2O(l)
Acid-base reaction
The brown/black CuO solid becomes a pale-blue solution of CuSO4
Lab 3: Chemical Reactions of Copper
What happens when you add elemental zinc to a solution of copper (II) sulfate? Write the reaction and what reaction type it is.
Zn(s) + CuSO4(aq) → Cu(s) + ZnSO4(aq)
Redox reaction
Blue solution of CuSO4 becomes colorless zinc (II) sulfate with reddish-brown deposit of elemental copper. Add 6M HCl to react with excess zinc. Wait for the H2 gas bubbles to dissipate before extracting Cu(s)
Lab 4: Spectrophotometric Analysis of Copper using Bathocuproine
Purpose/overview?
Determine the purity of a sample of elemental copper.
Lab 4: Spectrophotometric Analysis of Copper using Bathocuproine
Describe the reason behind the color change
Bathocuproine very specifically forms a yellow complex with copper (I) ions. It does not form complexes with other ions, even with copper (II)
Lab 4: Spectrophotometric Analysis of Copper using Bathocuproine
Calculating the molarity of Cu for the known samples.
Ex: using a 10 microgram/mL copper standard solution. The color standard was made using 2.0 mL of the Cu known standard solution and then diluted to total volume of 25 mL
microgram Cu/mL = (2 mL/25 mL)(10 microgram/mL) = 0.8 microgram Cu/mL
Molarity of Cu = (0.8 × 10^-6 g Cu/mL) (1000 mL/1 L)(1 mol Cu/63.55 g) = 1.26 × 10^-5 M
Lab 4: Spectrophotometric Analysis of Copper using Bathocuproine
Calculate the value of a (molar absorptivity) for a 1.26 × 10^-5 M Cu solution. The absorbance reading for this solution is 0.143 A. Include units.
Use Beer’s Law: A=abC
Thus, a=A/bC, where a is molar absorptivity, b = path length (here, b = 1 cm), C = molarity of solution, and A = absorbance.
Thus, a = (0.143)/(1 cm * 1.26 × 10^-5 M) = 1.13 × 10^4 (1/cm M)
Lab 4: Spectrophotometric Analysis of Copper using Bathocuproine
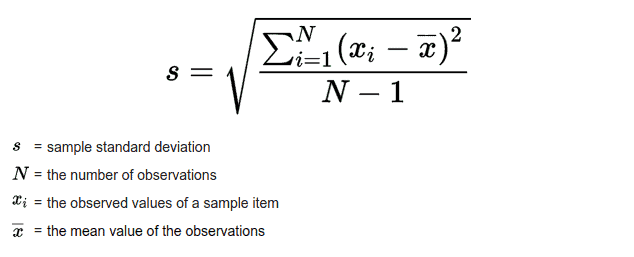
Equation for standard deviation?
Ex: Find standard deviation for the dataset: 1, 1, 2, 5, 4, 3
mean = 2.667
std. dev = 1.63
Lab 4: Spectrophotometric Analysis of Copper using Bathocuproine
Determination of copper content from calibration curve for unknown.
The average value of a = 1.19 × 10^4 (1/cm M)
A for unknown = 0.932
Plot absorbance on the y axis vs. micrograms Cu/mL on the x axis.
A=abC, b = 1 cm, values of A and a are given
Solve for C, and you will get C = 7.73 × 10^-5. Then, you can convert that value to micrograms/mL (4.91 micrograms/mL)
You could also find the slope of the plot. Since A increases with increasing concentration of copper (positive slope), you would expect [Cu] = 0 to have A = 0. Thus, just set 0.932 = (slope in mL/micrograms) x [Cu] and solve for [Cu], which will be in micrograms/mL
Lab 4: Spectrophotometric Analysis of Copper using Bathocuproine
It is determined that the content of copper unknown from calibration curve is 4.91 microgram/mL. What is total unknown copper in the 25 mL volumetric flask?
122.75 micrograms
Lab 4: Spectrophotometric Analysis of Copper using Bathocuproine
It is determined that the total unknown copper in the 25 mL flask is 122.75 micrograms. What is the total unknown copper in the second diluted, 100 mL flask
5 mL of the solution in the second diluted 100 mL flask was used to make the solution in the 25 mL flask
122.75 micrograms/5 mL x 100 mL = 2455 micrograms
Lab 4: Spectrophotometric Analysis of Copper using Bathocuproine
It is determined that the total unknown copper in the second diluted, 100 mL flask is 2455 micrograms. What is the total unknown copper (in g) in the 1st 100 mL flask?
10 mL of the solution in the 1st 100 mL flask were used to make the solution in the second diluted, 100 mL flask.
2455 micrograms/10 mL x 100 mL and convert to grams = 0.02455 g
Lab 4: Spectrophotometric Analysis of Copper using Bathocuproine
It is determined that the content of copper unknown from calibration curve is 4.91 microgram/mL. What is the % of Cu in the original sample (original mass = 0.0248 g)
Total copper in first flask was 0.0246 g. Divide this by the original mass and multiply by 100%.
99%
Lab 7: Synthesis of Aspirin
Purpose/overview?
To perform an esterification reaction where aspirin is produced. Then, to test the aspirin for contaminants/unreacted product.
Lab 7: Synthesis of Aspirin
Reaction to form aspirin
Salicylic acid + acetic anhydride → acetyl-salicylic acid (aspirin) + acetic acid
Salicylic acid contains a phenol group (benzene ring + OH). Aspirin does not
Reaction is catalyzed by concentrated mineral acid (like H3PO4)
Lab 7: Synthesis of Aspirin
How to test for contaminants?
To test for contaminants like starch (used as a binder for commercial aspirin tablets), you should add I2-KI
Lab 7: Synthesis of Aspirin
Why is a color observed when an aspirin tablet is tested with I2-KI?
The I2-KI reacts with starch in the tablet. This creates a starch-iodine complex, which has a very intense, deep-blue color. (Solid starches turn black when I2 is added)
Lab 7: Synthesis of Aspirin
How to test for unreacted starting material?
Unreacted starting material (salicylic acid) contains a phenol group, whereas aspirin does not. Adding iron (III) nitrate causes aspirin with unreacted salicylic acid to turn a deep purple color.
Lab 7: Synthesis of Aspirin
Why would a color be observed if iron (III) nitrate were added to commercial aspirin
Unreacted starting material (salicylic acid) contains a phenol group, whereas aspirin does not. Adding iron (III) nitrate causes aspirin with unreacted salicylic acid to turn a deep purple color. This is because iron (III) ion reacts with phenols to form a coordination compound with a deep purple color. Aromatic compounds without phenol groups (aspirin) do not change color.
Lab 7: Synthesis of Aspirin
Determination of theoretical yield of aspirin based on 2 g of salicylic acid as the LR
Molar mass of salicylic acid = 138.12 g, and molar mass of aspirin is 180.158 g
1:1 ratio, so theoretical yield is 2.6 g of aspirin
Lab 7: Synthesis of Aspirin
How to calculate pKa of aspirin?
Dissolve aspirin in 50/50 ethanol and water. Then, add phenolphthalein and titrate with 0.1 M NaOH
Record the volume of NaOH required. Then, divide this volume by 2. Add this divided volume of 0.1 M HCl to the same flask. Then, calibrate pH meter with buffer of pH = 4. Measure pH of flask. This value will be your pKa (should be around 3.5 for aspirin)
Lab 7: Synthesis of Aspirin
Why isn’t accurate weight of aspirin needed for determination of pKa?
The method of determining pKa only requires an equal ratio of aspirin to its conjugate base, as well as a way to measure pH (like a pH meter). Thus, it does not matter if accurate weight is used, as using an indicator to determine the moles of NaOH needed to completely neutralize the aspirin is enough.
Lab 8: Determination of Ksp
Purpose/overview?
To determine Ksp of Ca(IO3)2 and study common ion effect
You can determine the amount of iodate ion in your solution by reacting one mol iodate with 6 moles of (S2O3)2-. This process produces I-.
Thus, use 0.025 M Na2S2O3 as the titrant, and use starch as an indicator. Starch reacts with I2 reversibly to form a dark colored solution. However, I2 is consumed in the reaction of (S2O3)2- with iodate, so once the iodate has been used up, the dark color disappears to become clear.
Lab 8: Determination of Ksp
It took 17.03 mL of 0.0250 M Na2S2O3 in order for the filtrate of Ca(IO3)2 (no common ions) to reach the endpoint. What is the value of Ksp?
It took 0.000426 moles of (S2O3)2-. Since six moles of (S2O3)2- reacts with one mole of iodate, the moles of iodate in the flask is 0.0000710 moles. These moles of iodate came from the 5 mL of filtrate added to the flask. Thus, the concentration of iodate in the original filtrate is 0.0142 M.
[Ca2+] in the filtrate is half of [(IO3)-] in the filtrate. Thus, [Ca2+] = 0.00710 M. This means Ksp = 1.44 × 10^-6
Lab 8: Determination of Ksp
It took 22.08 mL of 0.0250 M Na2S2O3 in order for the filtrate of Ca(IO3)2 (common ion solution of 0.01 M KIO3) to reach the endpoint. What is the value of Ksp?
It took 0.000552 moles of (S2O3)2-. Since six moles of (S2O3)2- reacts with one mole of iodate, the moles of iodate in the flask is 0.000092 moles. These moles of iodate came from the 5 mL of filtrate added to the flask. Thus, the concentration of iodate in the original filtrate is 0.0184 M.
The concentration of iodate in the original filtrate is the sum of iodate from dissolved Ca(IO3)2 AND iodate from the 0.01 M KIO3 solution. Thus, the iodate concentration from the dissolved Ca(IO3)2 is 0.0084 M.
[Ca2+] in the filtrate is half of [(IO3)-] in the filtrate that was from the dissolved Ca(IO3)2. Thus, [Ca2+] = 0.0042 M. The value of [(IO3)-] at equilibrium is still 0.0184 M. This means Ksp = 1.42 × 10^-6
Lab 8: Determination of Ksp
The values of Ksp for both the regular filtrate and the common ion filtrate should be the same. Why?
The value of Ksp can only be affected by temperature. The common-ion effect does not impact solubility by impacting Ksp. Rather, the common-ion effect shifts the equilibrium towards the left. The addition of KIO3 presents a common ion, iodate, that shifts the equilibrium left, decreasing the solubility of Ca(IO3)2.
Lab 8: Determination of Ksp
Correct value for Ksp of Ca(IO3)2
Ksp = 1.7 × 10^-6
Lab 9: Oxidation-Reduction Reactions of the Halogens
Purpose/overview?
To study the relative oxidizing strengths of halogens (Cl2, Br2, I2) and the relative reducing strengths of the halides (Cl-, Br-, I-)
Lab 9: Oxidation-Reduction Reactions of the Halogens
Color of:
Chlorine water
Bromine water
Iodine water
Chlorine water is colorless to yellow
Bromine water is yellow to red-brown
Iodine water is red-brown to brown
Lab 9: Oxidation-Reduction Reactions of the Halogens
Cyclohexane is:
The top layer
Cyclohexane layer color for the halogen may be different from their aqueous color
Lab 9: Oxidation-Reduction Reactions of the Halogens
Purpose of KMnO4?
MnO4- is an oxidizing agent that can oxidize some of the halides.
In acidic solutions, MnO4- is reduced to Mn2+ (colorless ion)
In basic solution, MnO4- is reduced to MnO2 (brown solid insoluble in water). Can also be reduced to (MnO4)2-, which is the green manganate ion
Lab 9: Oxidation-Reduction Reactions of the Halogens
Color of the halogens in cyclohexane
Cl2 is mostly clear
Br2 is yellow/orange
I2 is purple
Lab 9: Oxidation-Reduction Reactions of the Halogens
Do Cl- and Br2 react?
No reaction
Cyclohexane remains yellow/orange (color of Br2)
Lab 9: Oxidation-Reduction Reactions of the Halogens
Do Cl- and I2 react?
No reaction
Cyclohexane remains purple (color of I2)
Lab 9: Oxidation-Reduction Reactions of the Halogens
Do Br- and Cl2 react?
Cl2 + 2Br- → 2Cl- + Br2
Cyclohexane turns yellow/orange (color of Br2)
Lab 9: Oxidation-Reduction Reactions of the Halogens
Do Br- and I2 react?
No reaction
Cyclohexane remains purple (Color of I2)
Lab 9: Oxidation-Reduction Reactions of the Halogens
Do I- and Cl2 react?
Cl2 + 2I- → 2Cl- + I2
Cyclohexane turns purple (color of I2)
Lab 9: Oxidation-Reduction Reactions of the Halogens
Do I- and Br2 react?
Br2 + 2I- → 2Br- + I2
Cyclohexane turns purple (color of I2)
Lab 9: Oxidation-Reduction Reactions of the Halogens
Do Br-, KMnO4, and H2SO4 react?
10Br- + 2(MnO4)- + 16H+ → 5Br2 + 2(Mn)2+ + 8H2O
Cyclohexane becomes yellow/orange (color of Br2)
Lab 9: Oxidation-Reduction Reactions of the Halogens
Do I-, KMnO4, and H2SO4 react?
10I- + 2(MnO4)- + 16H+ → 5I2 + 2(Mn)2+ + 8H2O
Cyclohexane becomes a purple color (color of I2)
Lab 9: Oxidation-Reduction Reactions of the Halogens
Do I-, NaOH, and KMnO4 react?
I- + 6(MnO4)- + 6OH- → 6(MnO4)2- + (IO3)- + 3H2O
Cyclohexane becomes a green color (color of (MnO4)2-)
Lab 9: Oxidation-Reduction Reactions of the Halogens
Rank the halogens in terms of oxidizing strength
Cl2 > Br2 > I2
Lab 9: Oxidation-Reduction Reactions of the Halogens
Rank the halides in terms of reducing strength
I- > Br- > Cl-