Module 10 Online HW Review Bonus
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Which of the following is not an assumption of the kinetic-molecular theory?
All the gas particles in a sample have the same velocity.
3 multiple choice options
Which of the following instruments directly measures the pressure of a gas?
manometer
3 multiple choice options
What is the Celsius temperature of 100.0 g of chlorine gas in a 40.0-L container at 800. mm Hg?
91 °C
3 multiple choice options
When 8.21 L of C3H8 (g) burn in oxygen, how many liters of oxygen are consumed? All gas volumes are measured at the same temperature and pressure but not at STP.
41.0 L
3 multiple choice options
Which one of the following is not used to describe the condition of an ideal gas?
polarity
3 multiple choice options
A gas sample of argon, maintained at constant temperature, occupies a volume of 500. L at 4.00 atm. What is the new volume if the pressure were charged to 8.0 atm?
250 L
3 multiple choice options
How many grams of the excess reagent are left over when 6.00 g of CS2 gas react with 10.0 g of Cl2 gas in the following reaction?
CS2(g) + 3 Cl2(g) → CCl4(l) + S2Cl2(l)
2.42 g
3 multiple choice options
The SI unit for pressure is:
pascal
3 multiple choice options
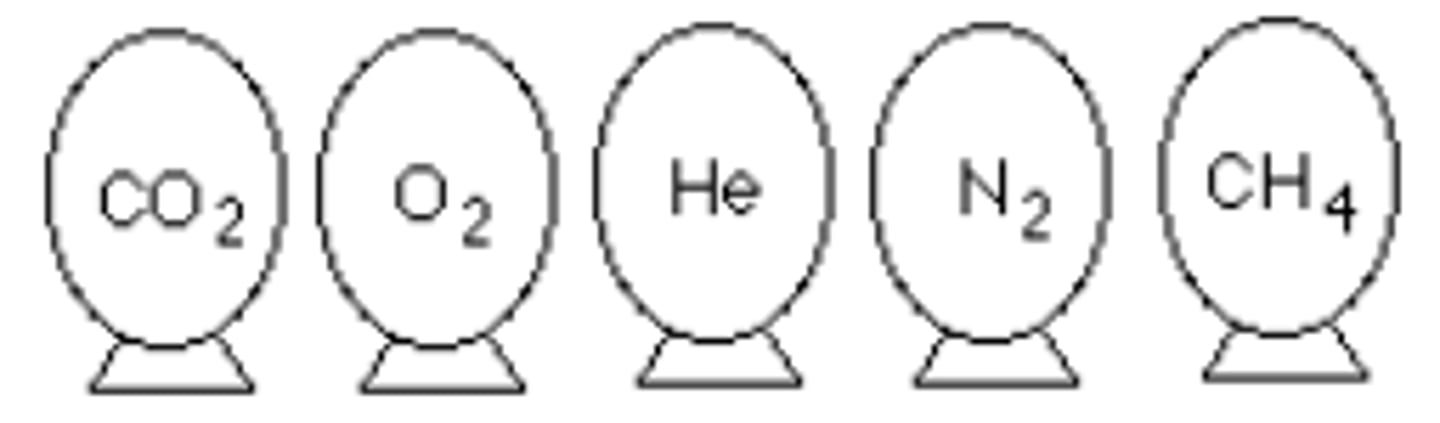
Represented below are five identical balloons, each filled to the same volume at 25°C and 1.00 atm pressure with the pure gas indicated. Which balloon contains the gas that would be expected to deviate most from the behavior of an ideal gas?
CO2
3 multiple choice options
A gas is under 25 atm of pressure occupies 35.0 L at 127 oC. What is the volume of the gas at 1.00 atm and 0 oC?
597 L
3 multiple choice options
What is the resulting temperature of the gas if 200. mL of a gas sample at 27 oC expands to 500. mL?
All of these
3 multiple choice options
The volume of 350. mL of gas at 25°C is decreased to 125 mL at constant pressure. What is the final temperature of the gas?
-167 °C
3 multiple choice options
A gas occupies 22.4 L at STP and 14.5 L at 100°C and 2.00 atm pressure. How many moles of gas did the system gain or lose?
0.05 moles lost
3 multiple choice options
What is the number of moles in 500. L of He gas at STP?
22.0 mol
3 multiple choice options
What pressure would be exerted by 76 g of fluorine gas in a 1.50 L vessel at -37 oC?
26 atm
3 multiple choice options
When 0.400 mole of potassium reacts with excess water at standard temperature and pressure as shown in the equation above, the volume of hydrogen gas produced is:
2 K (s) + 2 H2O (l) → 2 K+ (aq) + 2 OH- (aq) + H2 (g)
4.48 L
3 multiple choice options
A sample of gas has a volume of 0.600 L at a temperature of 30.oC and a pressure of 0.800 atm. What is the number of moles in this sample?
0.0200 mol
3 multiple choice options
Hydrogen gas is collected over water at 24 °C. The total pressure of the sample is 755 mmHg. At 24 °C, the vapor pressure of water is 22 mmHg. What is the partial pressure of the hydrogen gas?
733 mmHg
3 multiple choice options
Three identical flasks contain three different gases at standard temperature and pressure. Flask A contains CH4, flask B contains CO2, flask C contains N2. Which flask contains the largest number of molecules?
All flasks contain the same number of molecules.
3 multiple choice options
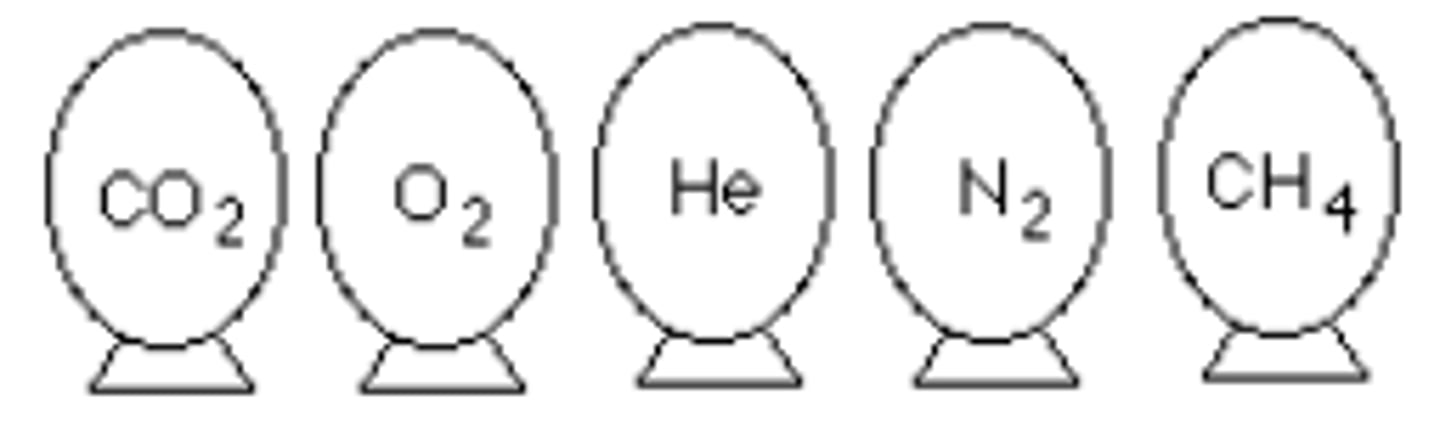
Represented below are five identical balloons, each filled to the same volume at 25°C and 1.00 atm pressure with the pure gas indicated. Which balloon contains the greatest mass of gas?
CO2
3 multiple choice options
An "empty" aerosol can at 25°C still contains gas at 1.00 atmosphere pressure. If an "empty" can is thrown into a 475°C fire, what is the final pressure in the heated can?
2.51 atm
3 multiple choice options
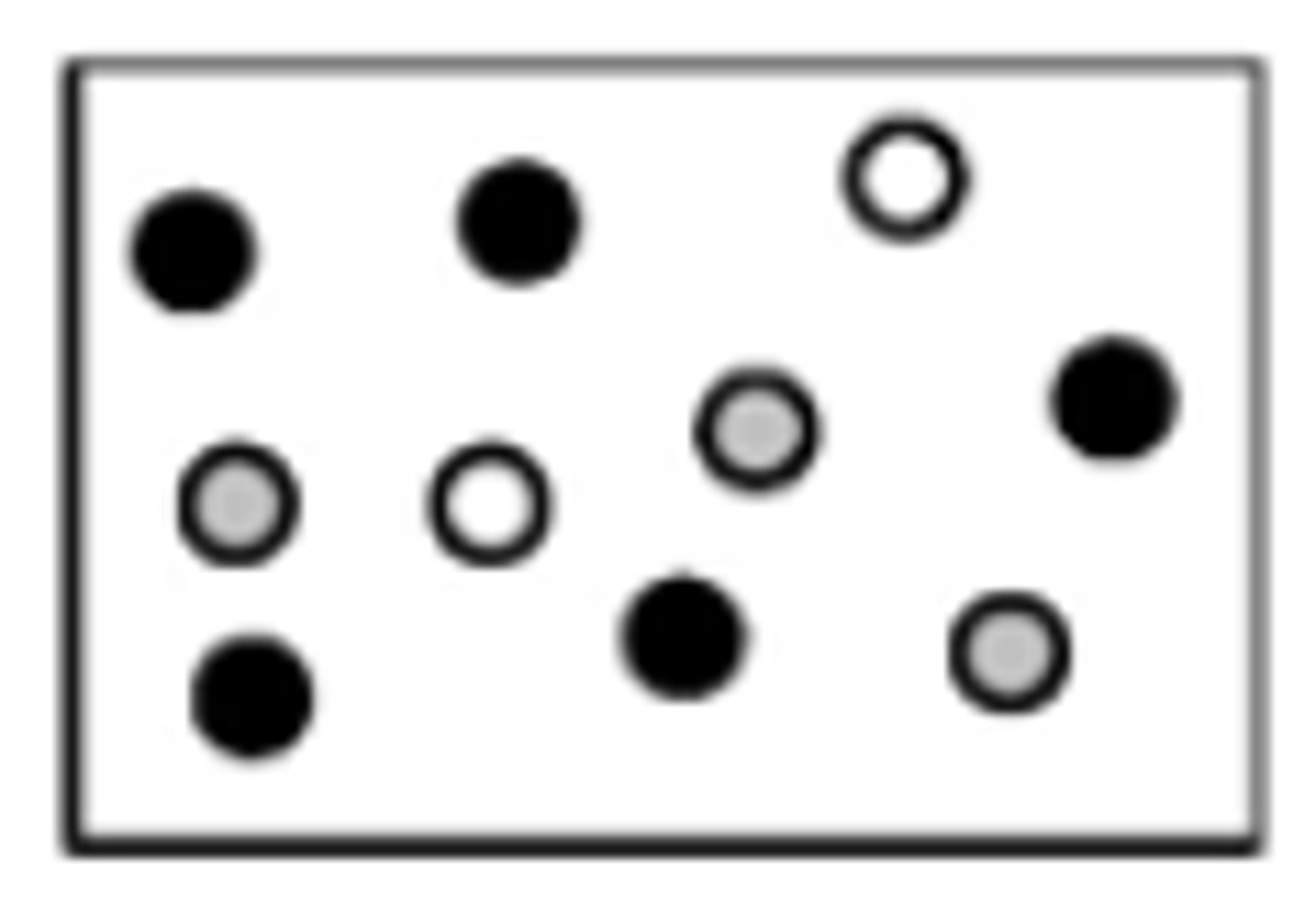
In the diagram below, helium atoms are represented by unshaded spheres, neon atoms by gray spheres, and argon atoms by black spheres. If the total pressure in the container is 900. mm Hg, what is the partial pressure of helium?
180. mmHg
3 multiple choice options
A gas bottle contains 0.650 mol of gas at 730. mmHg pressure. If the final pressure is 1.15 atm, how many moles of gas were added to the bottle?
0.128 mol
3 multiple choice options
Which statement is false?
The density of a gas is constant as long as its temperature remains constant.
3 multiple choice options
Automobile tires are typically inflated to about 30 pounds of pressure per square inch. What is the typical air pressure of a tire in kPa?
2.1 × 10^2 kPa
3 multiple choice options
A 1.00 L flask contains nitrogen gas at 25°C and 1.00 atm pressure. What is the final pressure in the flask if an additional 2.00 g of N2 gas is added to the flask and the flask cooled to -55°C?
2.01 atm
3 multiple choice options
A balloon contains 56 mmHg N2, 134 mmHg He, 423 mmHg H2 and some amount of O2 at 739 mmHg. What is the partial pressure of O2?
126 mmHg
3 multiple choice options
Pressure is defined as:
force divided by unit area.
3 multiple choice options
When 6.0 mol of oxygen are confined in a 36 L vessel at 196 oC, the pressure is 8.0 atm. What is the new pressure for oxygen expands at constant temperature to fill 48 L?
6.0 atm
3 multiple choice options
A gaseous mixture containing 7.00 moles of nitrogen, 2.50 moles of oxygen, and 0.500 mole of helium exerts a total pressure of 0.900 atm. What is the partial pressure of the nitrogen?
0.63 atm
3 multiple choice options
A sample of a gas ( 3.9 mol ) at 1.3 atm is expanded at constant temperature from 1510 L to 15 L. The final pressure is ________ atm.
131
2 multiple choice options