Integrated Rate Law
0.0(0)
0.0(0)
Card Sorting
1/13
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
1
New cards
Units of the Rate Constant (k)
M^1-n⋅t^-1
2
New cards
Units of k for Zero Order
M^1⋅t^-1
3
New cards
Units of k for First Order
M^0⋅t^-1
4
New cards
Units of k for Second Order
M^-1⋅t^-1
5
New cards
Half Life for Zero Order
t1/2 = [A]°/2k
6
New cards
Half Life for First Order
t1/2 = ln2/k
7
New cards
Half Life for Second Order
t1/2 = 1/k⋅[A]°
8
New cards
IRL Equation for Zero Order
[A]f = -k⋅t + [A]°
9
New cards
IRL Equation for First Order
ln[A]f = -k⋅t + ln[A]°
10
New cards
IRL Equation for Second Order
1/[A]f = k⋅t + 1/[A]°
11
New cards
IRL Graph for Zero Order
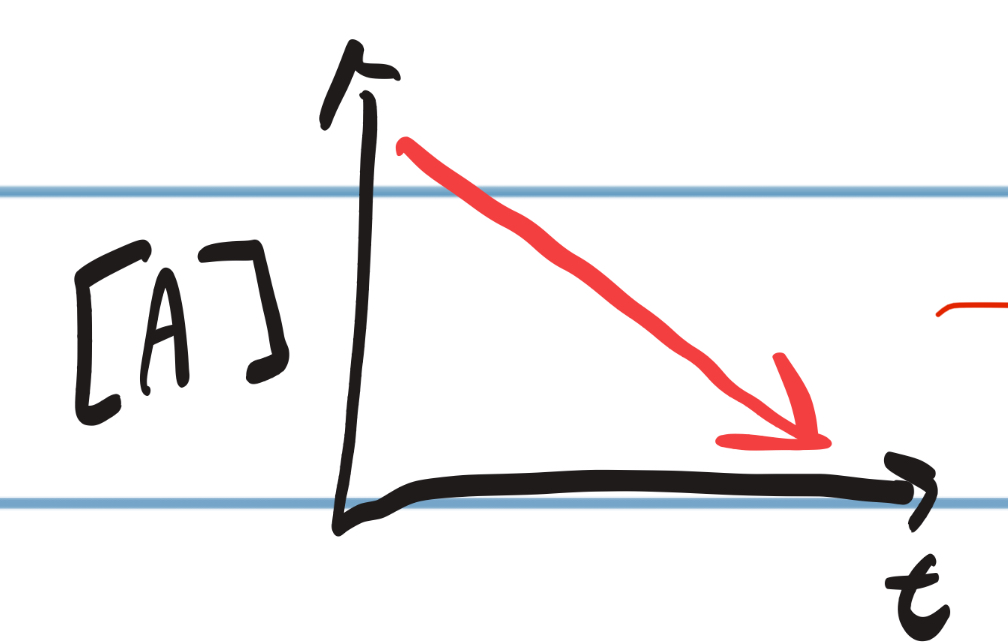
12
New cards
IRL Graph for First Order
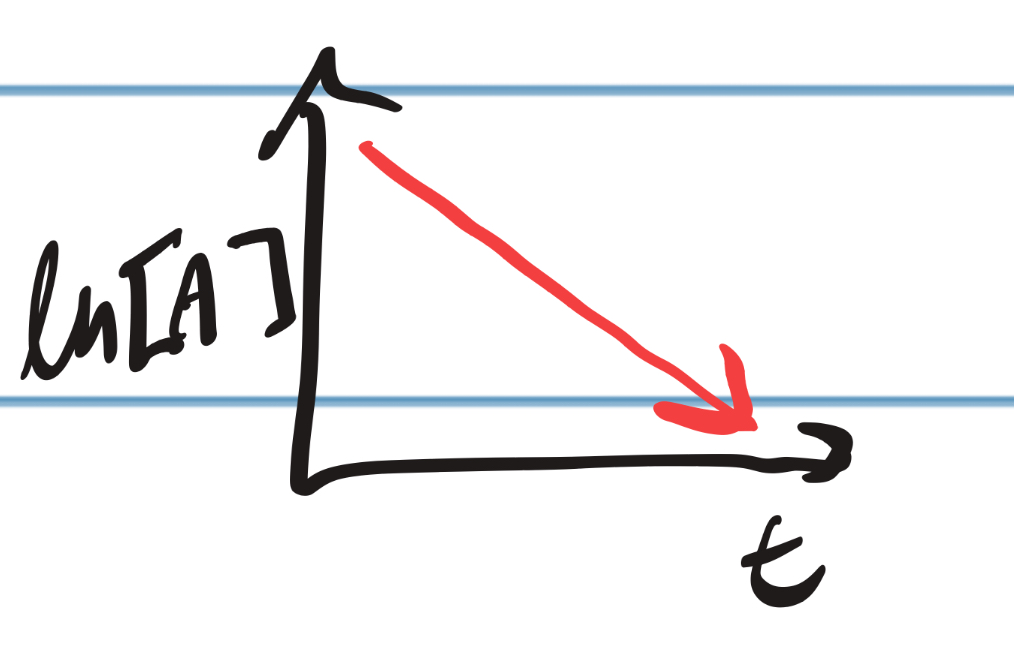
13
New cards
IRL Graph for Second Rate Law
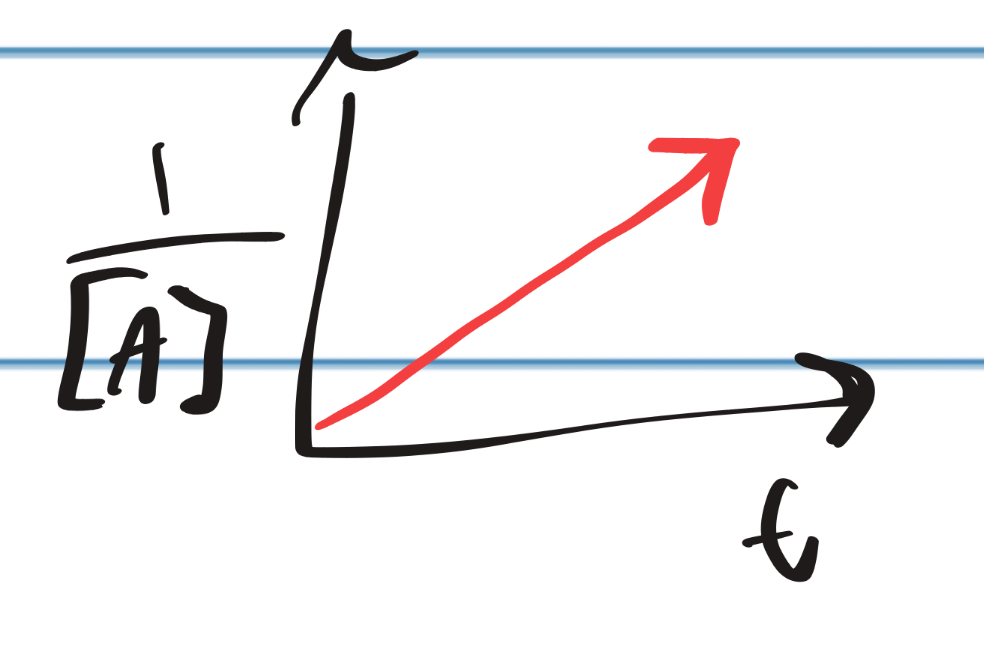
14
New cards
Nth Order Reaction Equation
t1/2 = 1/k(n-1)⋅2^(n-1)-1/[A]°^(n-1)