The third Law of thermodynamics/entropy principle
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
what is the third law of thermodynamics?
the entropy of a pure crystalline substance at absolute zero temperature is zero
what is absolute entropy?
entropy relative to the entropy of a pure crystalline substance at absolute zero (which is zero)
what is the order of entropy of the three main states of matter?
Gas > Liquid > Solid
what is the increase of entropy principle?
entropy is always increasing
why is entropy generated during irreversible processes?
due to the presence of irreversibilties
how can you identify the reversibility of a system based off of entropy generated?
Entropy generated < 0 IMPOSSIBLE
Entropy generated = 0 REVERSIBLE
Entropy generated > 0 IRREVERSIBLE
what is the equation of entropy for an isothermal case?
δQ/T
what is the basic energy direction convention?
Q in +
Q out -
W in -
W out +
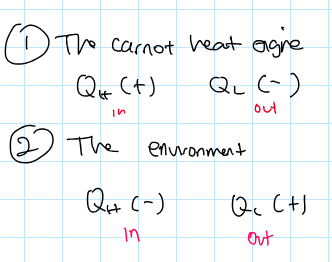
What is the sign convention for a carnot heat engine and for the environment?
when is entropy conserved?
entropy is only conserved during IDEALISED REVERSIBLE PROCESSES and increases during all actual processes
how can you work out quality from steam tables using entropy?
S = S(f) + xS(fg)
what is an isentropic process?
entropy change = 0