Chem Exam 1
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Last updated 4:32 PM on 5/12/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
1
New cards
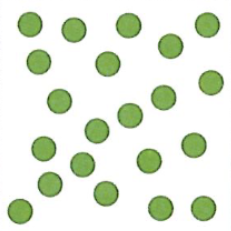
Element
Pure substance comprised of one type of atom
2
New cards
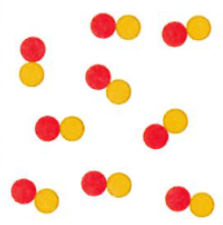
Compound
Pure substance comprised of two or more types of atoms
3
New cards
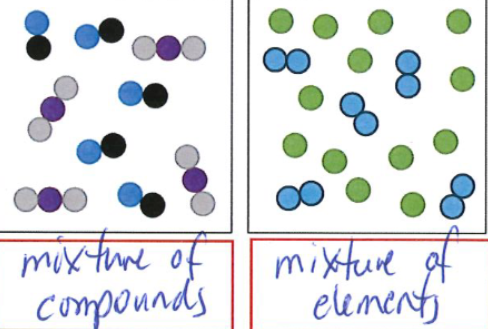
Mixture
two more pure substances
4
New cards
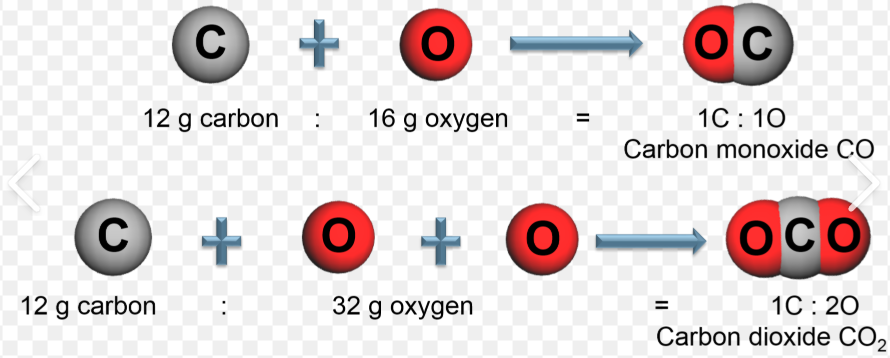
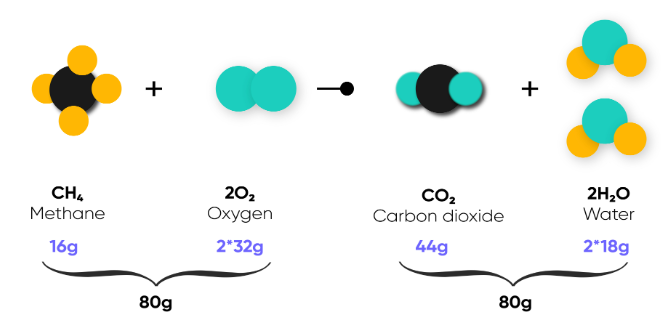
Law of concentration of Mass
States that mass is neither created nor destroyed in a chemical reaction
The mass of reactants equals the mass of products.
Consistent with the idea that matter is comprised of tiny indestructible bits of matter
5
New cards
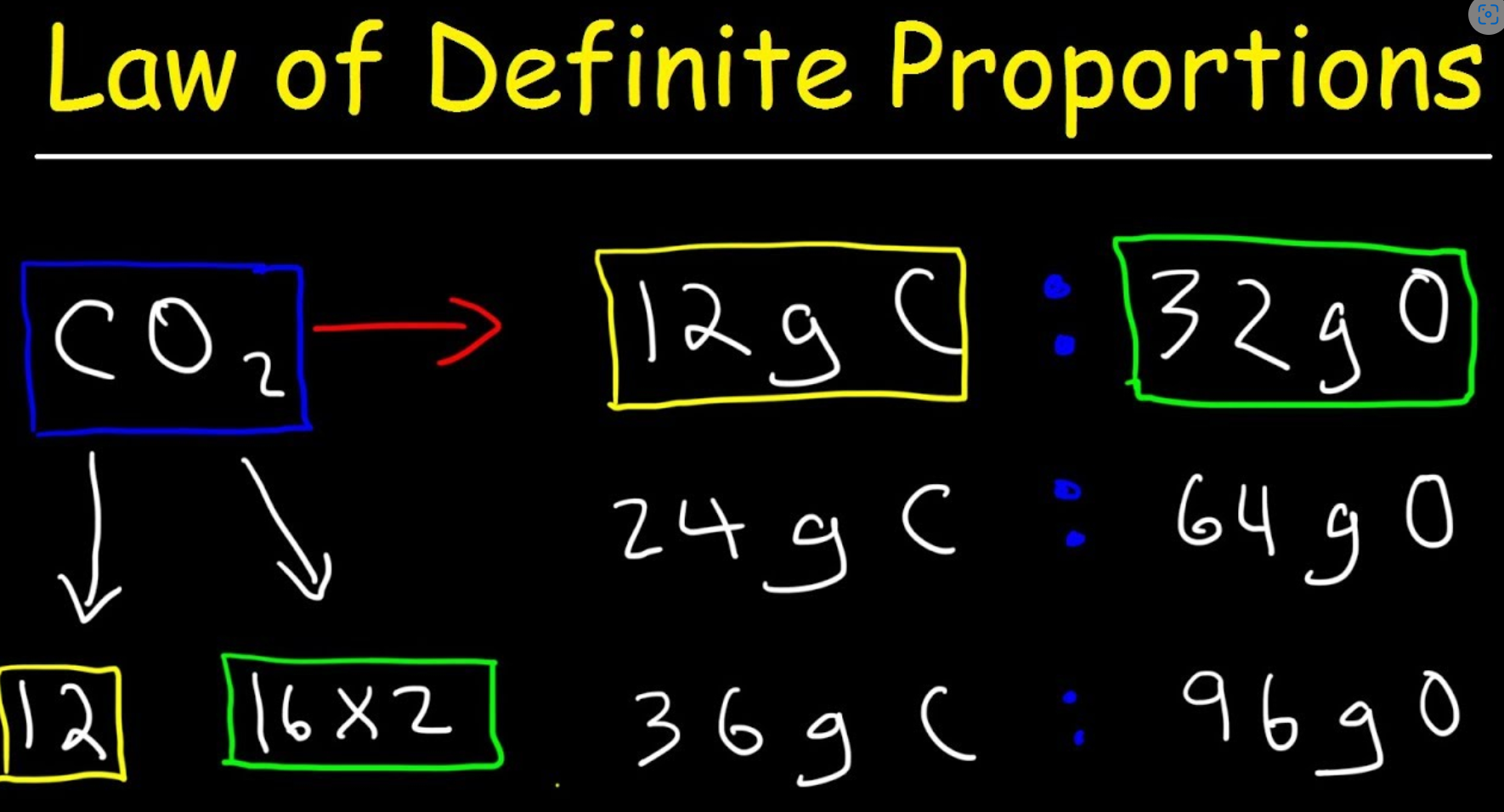
Law of Definite Porportions
A chemical compound contains the same proportion of elements
All atoms of each type are identical and combine in specific ratios.
6
New cards
Law of Multiple Proportions
Compounds are made of atoms in fixed, small-number ratios - Dalton