CHEM 20A Final
1/152
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
153 Terms
Extensive Property
a property that depends on the size of the substance
ex: volume, mass
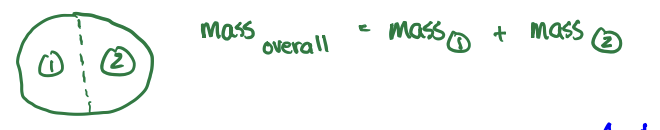
Intensive Property
a property that is independent of the size of the substance
ex: temp, density
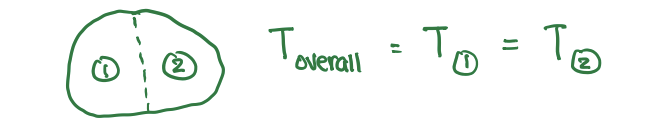
Law of Conservation of Mass (Lavoisier)
Experiment: decomposition of mercury oxide
Significance: not creating or destroying building blocks, but rearranging in chemical reactions; you combine them in different ways and it comes out with a different property
Law of Definite Proportion (Berthollet vs. Proust)
Experiment: Lavoisier’s experiment many times and measure [mass of gas/mass of mercury oxide]
Berthollet said % mass of gas is not fixed; it can vary over a range
Proust said, in a given chemical compound, the proportions by mass of elements that compose it are fixed; variations due to error or impurities
Significance: building blocks combine in specific ratios (ONE COMPOUND) to form different compounds
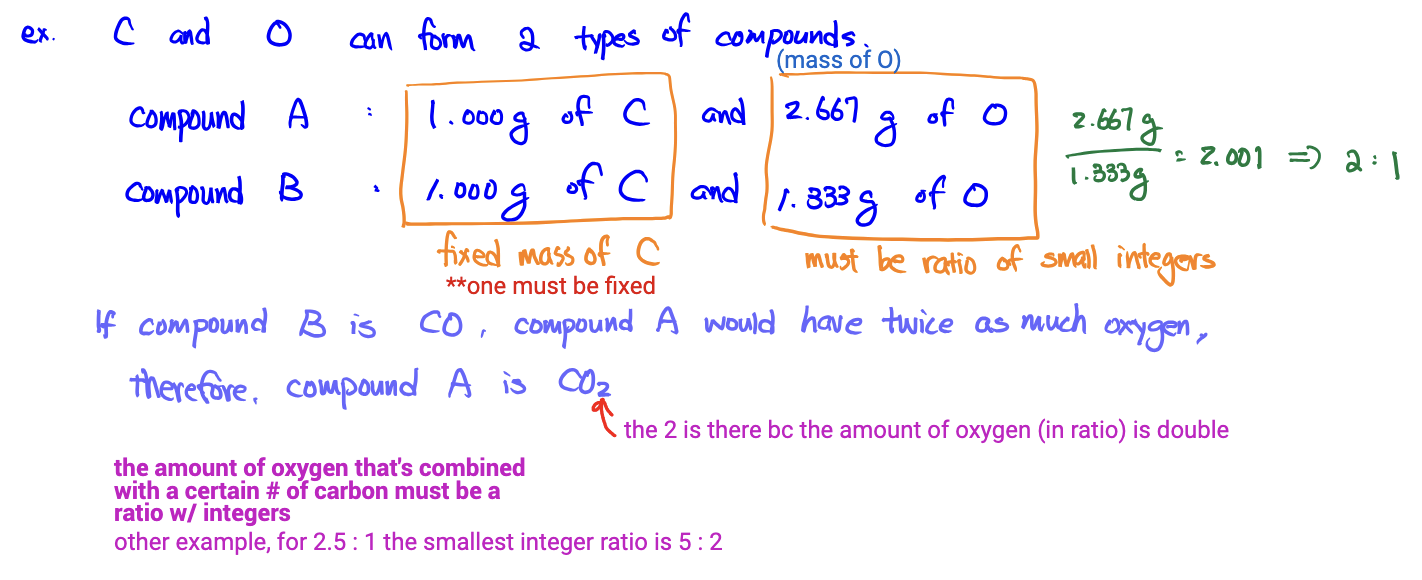
Law of Multiple Proportions
When two elements form a multiple compounds (DIFFERENT COMPOUNDS WITH SAME ELEMENTS), the masses of one element that combine with a fixed mass (1g) of the other element are in the ratio of small integers to each other.
*note: when solving, one element should be fixed
Dalton’s Atomic Theory of Matter (1808)
Significance: summarized the conservation of mass and definite proportion; coined “atom”
The two main things he got wrong:
He said that atoms are indestructible/indivisible
Can be further broken down to electrons, protons, and neutrons
He assumed the identity of the atom is determined by the mass
Not true because of isotopes
Law of Combining Volumes (Gay-Lussac)
The ratio of the volumes of any pair of gases in a gas phase chemical reaction (at the same temperature and pressure) is the ratio of simple integers.
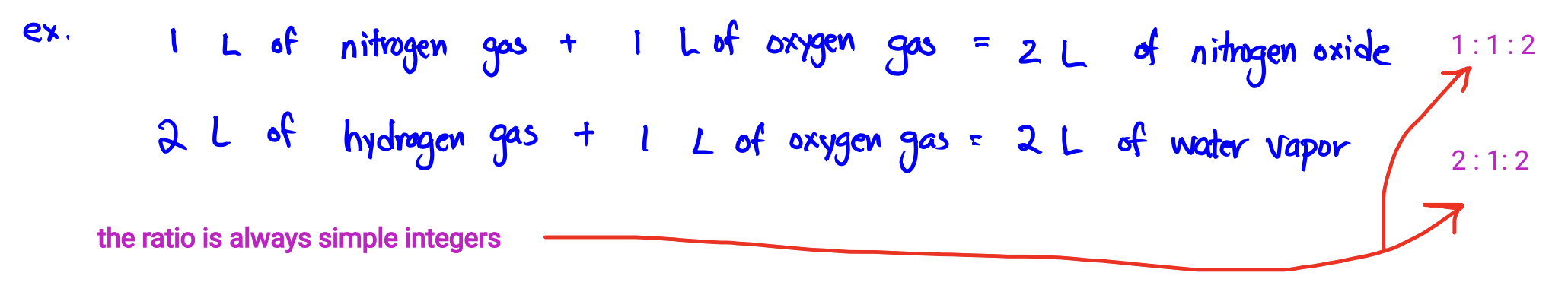
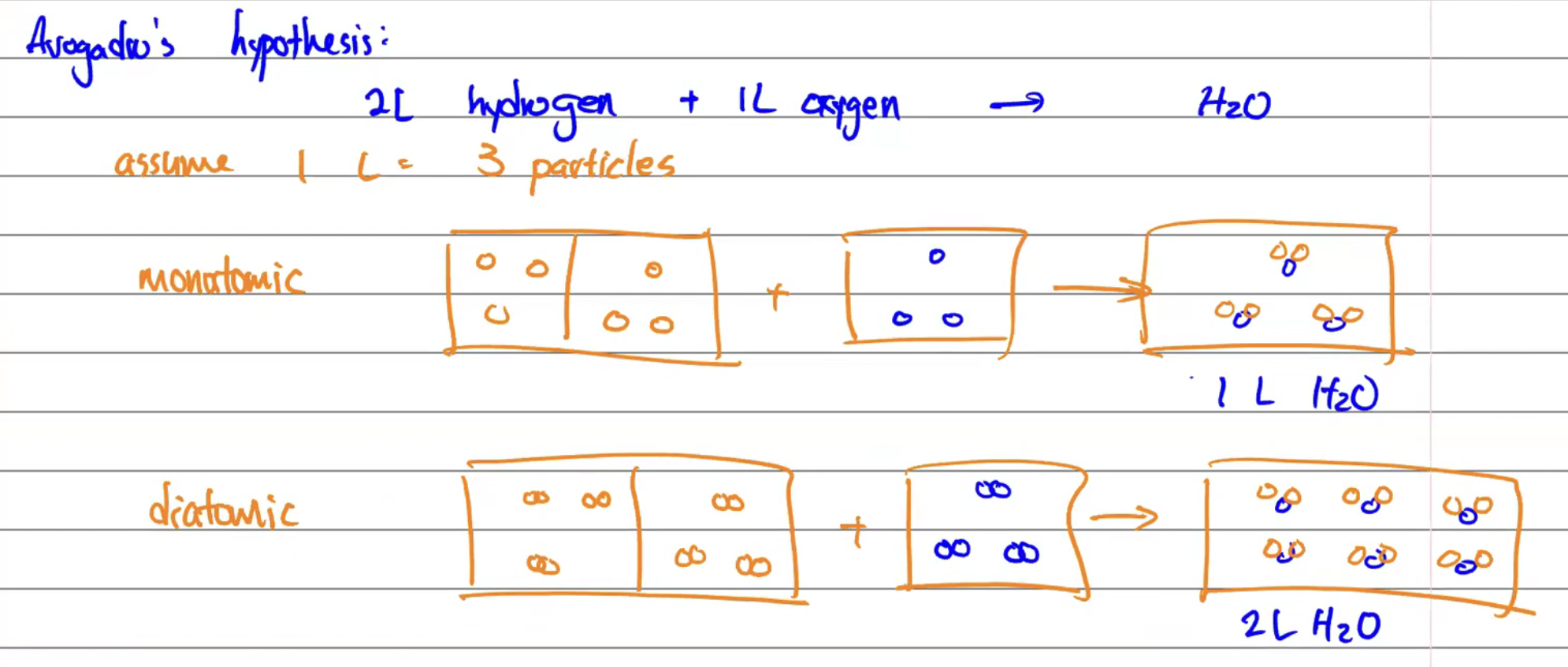
Avogadro’s Hypothesis
Equal volumes of different gases at the same temperature and pressure contain equal numbers of particles.
Experiment: 1 L of N + 1 L of O = 2 L of NO
The assumption would be that it would = 1 L NO, but since it’s 2 L NO, the hypothesis suggests N and O are diatomic molecules
Mole
Conceptualization: a unit for an amount of a substance, kinda like how a pair = 2 and a dozen = 12
Formula: 1 mol of atoms = 6.0221 × 10^23 atoms
Avogadro’s Number: 6.0221 × 10^23
If I divide mass (g) by the molar mass, I get mol of molecules
Avogadro’s Number
6.0221 × 10^23 atoms
Relationship between average atomic mass and mole
avg. atomic mass in amu = 1 mol of [element] atoms
![<p>avg. atomic mass in amu = 1 mol of [element] atoms</p>](https://knowt-user-attachments.s3.amazonaws.com/812f5fbf-ebae-437c-9f2f-3f477bdfd2ba.png)
Molar Mass of an Element
molar mass of an element = mass of one mole of its atoms (atomic mass)
Molecular Weight (molar mass of a compound)
molar mass of a compound = mass of 1 mole of its molecules
To find the molar mass of a compound, add up the avg. atomic mass of all the atoms in a molecule
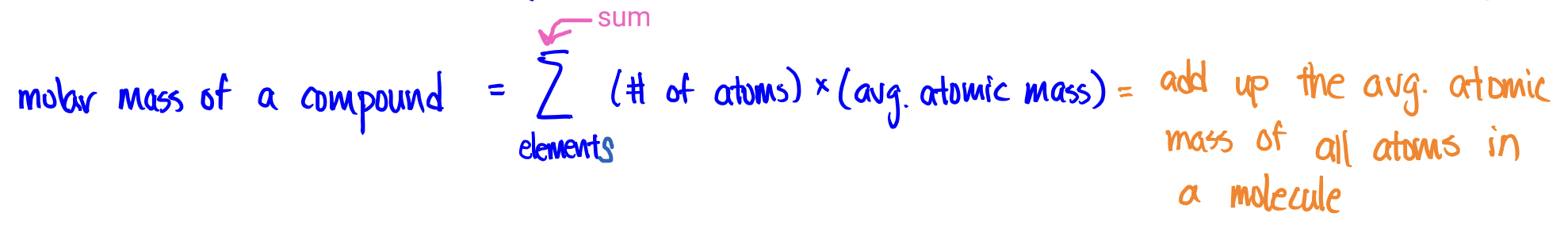
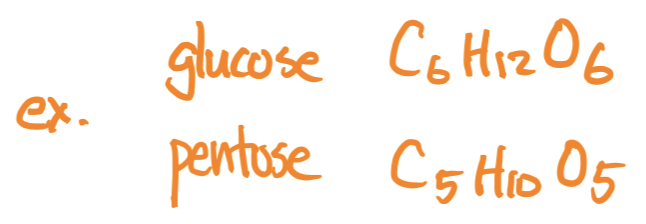
Molecular Formula
the actual number of atoms in a molecule
Empirical Formula
the simplest whole number ratio of atoms
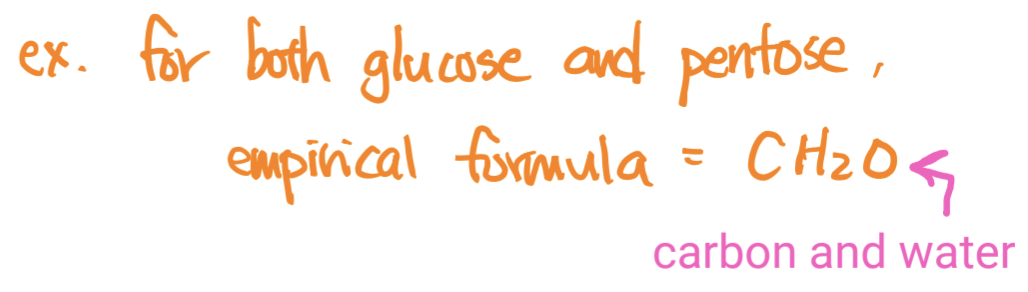
Percent Mass Composition
(mass of element in a compound)/(mass of a molecule)

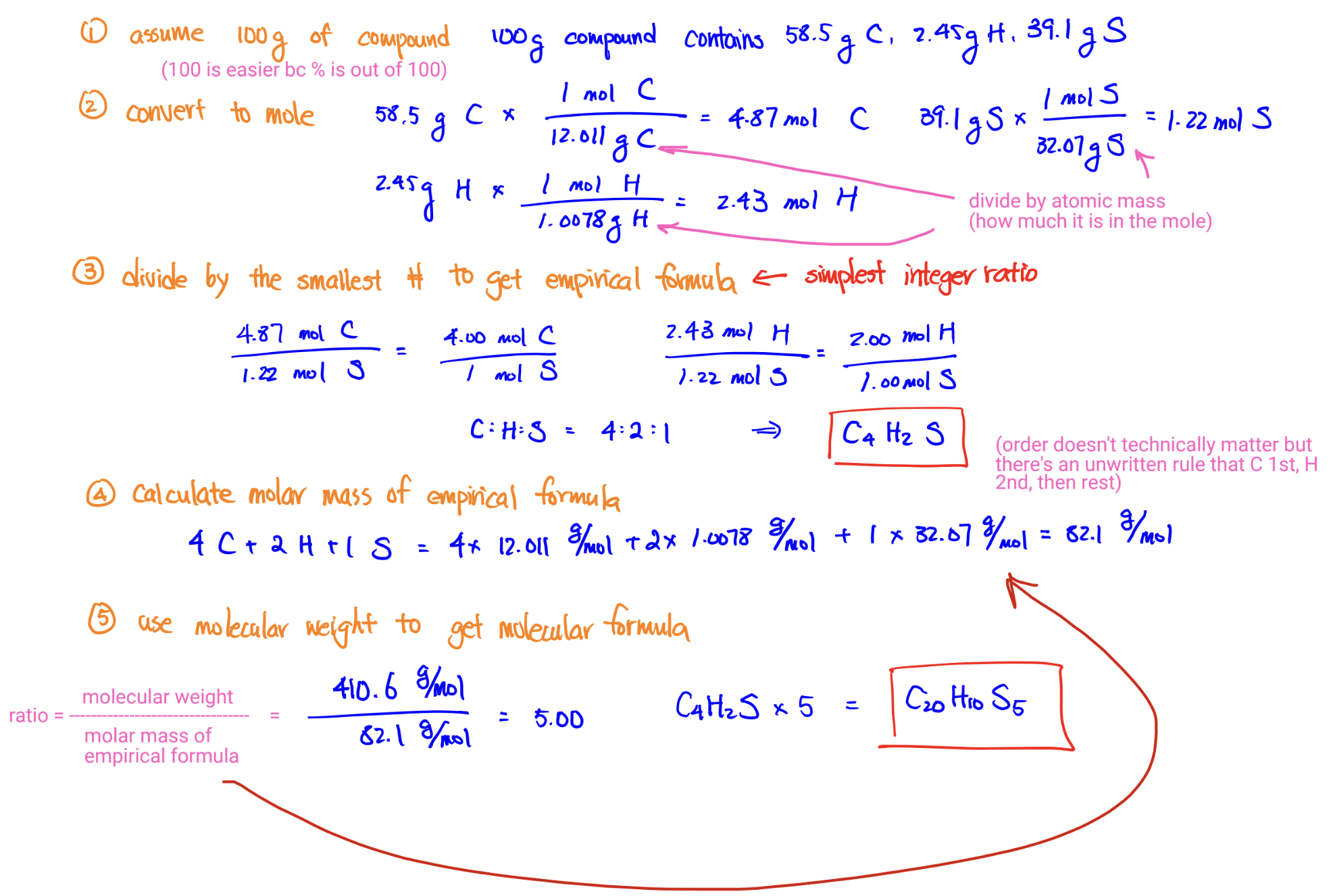
Empirical Formula from Percent Mass Composition
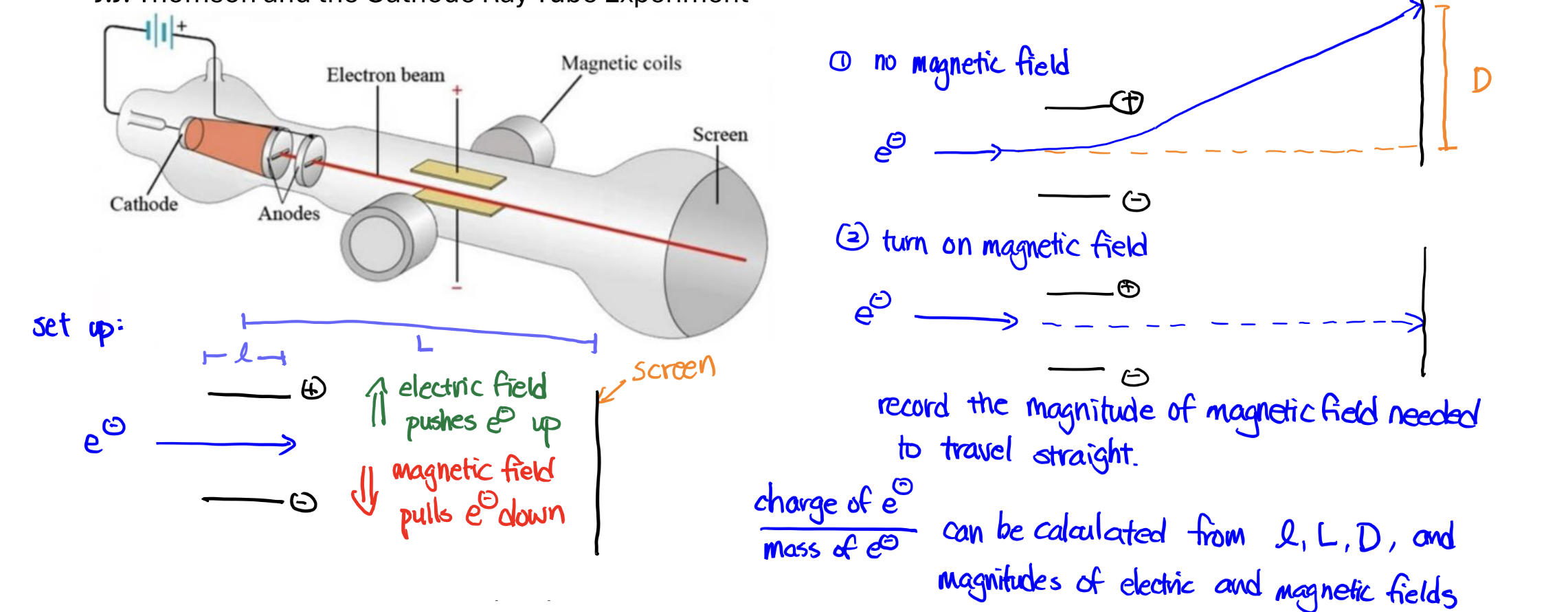
Cathode Ray Tube Experiment
Experiment: shoot a ray of electrons through a tube with an electric and magnetic field affecting the ray; electric directs the path, and changing the magnetic field can make it go straight (the light can curve without magnetic field, the distance = D)
Significance: charge-to-mass ratio
Oil Drop Experiment
Experiment: oil drop given a charge, and when it falls (gravity pulls it down), the negatively charged drop is suspended in air by changing the magnitude of the electric field
Significance: charge of e- (it’s 1.6 × 10^-19)
(charge can be calculated from the mass of the oil drop and the magnitude of the electric field needed to balance g-force)
If there are multiple e-s, the difference in charges has to be an integer multiple of the charge of the e-, and the smallest difference is the charge of the e-
Gold Foil Experiment
Before, the Plum-Pudding Model said an atom was made up of a positively charged “pudding” with e-s evenly spread out because they repel.
However, when a sheet of gold was shot with positively charged particles, there were some that were deflected/bounced back.
This suggested that atoms have a core (the nucleus) that’s dense and positively charged
Significance: discovery of a nucleus
Mass Spectrometry
A magnetic field bends the path of e-s in a beam, and it was seen that some atoms of the same element were bent differently. Lighter atoms bend more, and heavier atoms bend less.
Significance: atoms of the same element CAN have different masses; discovery of isotopes
Calculation of Average Atomic Mass
SUM of all isotopes’ [(mass x abundance)]
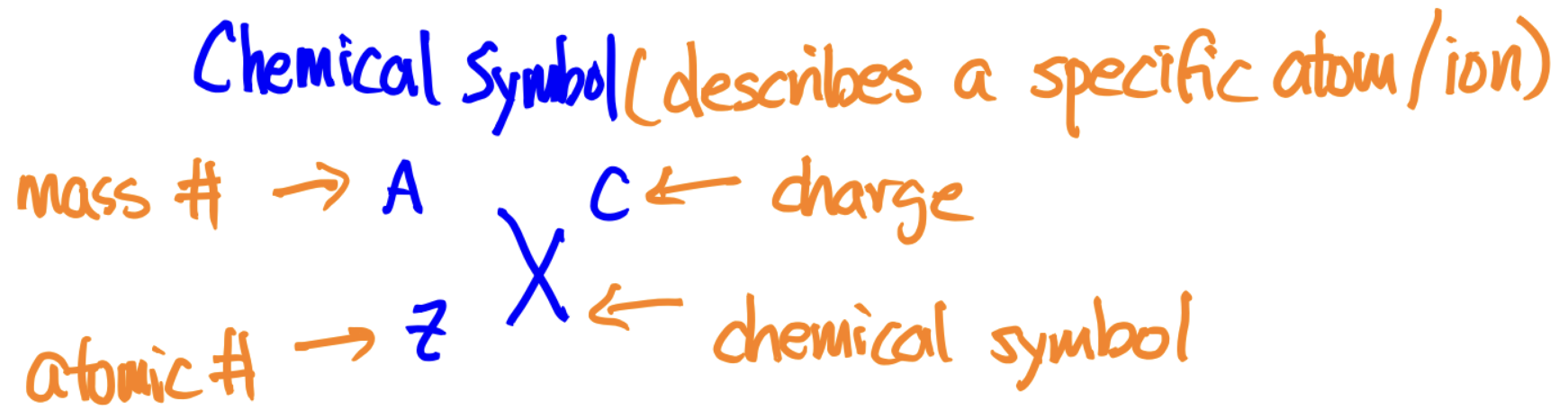
The mass of each is denoted by the superscript before the chemical symbol (the superscript after is the charge)
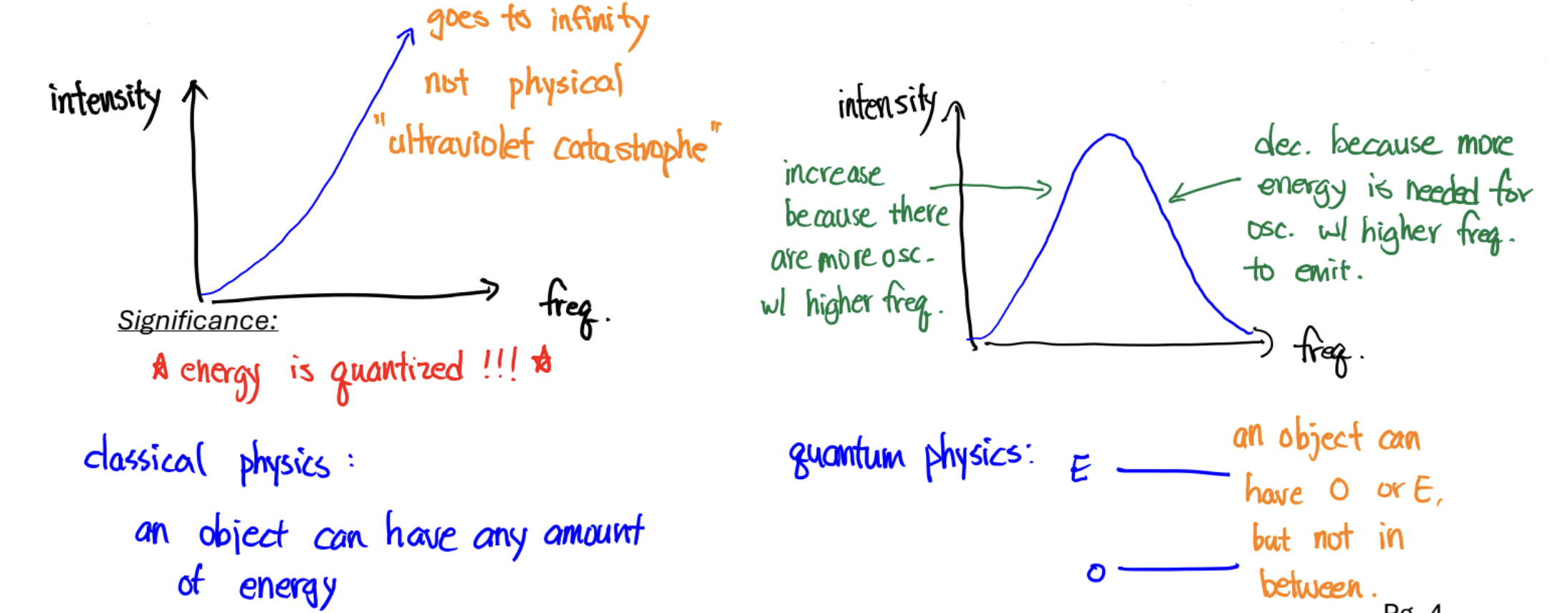
Blackbody Radiation
Significance: energy is quantized
For a blackbody (object), as temp increases, the frequency of its most intense color increases. This is because more energy is needed to turn on the light bulbs of higher frequency (energy to turn on/# of light bulbs is proportional to freq).There is more energy as temperature increases, so the higher frequency bulbs can be turned on. The “lighting up” of the higher frequency bulbs means the most intense color also shifts to a higher frequency.
Classical Physics vs. Planck (and quantum physics)
Classical Physics:
energy shared equally to all light bulbs
# of light bulbs proportional to its frequency
it can go up to infinity/objects can have any amount of energy
HOWEVER: not physical (doesn’t actually happen); “ultraviolet catastrophe“
LBs must have energy to give off electromagnetic radiation & # of LBs proportional to freq…
Planck:
energy is not distributed equally
energy given to each light bulb is proportional to the frequency
more energy needed to give energy to LB w/ high freq
fewer high freq LB can emit
ENERGY QUANTIZED!!!
quantum physics: object can have E or O but no b/w
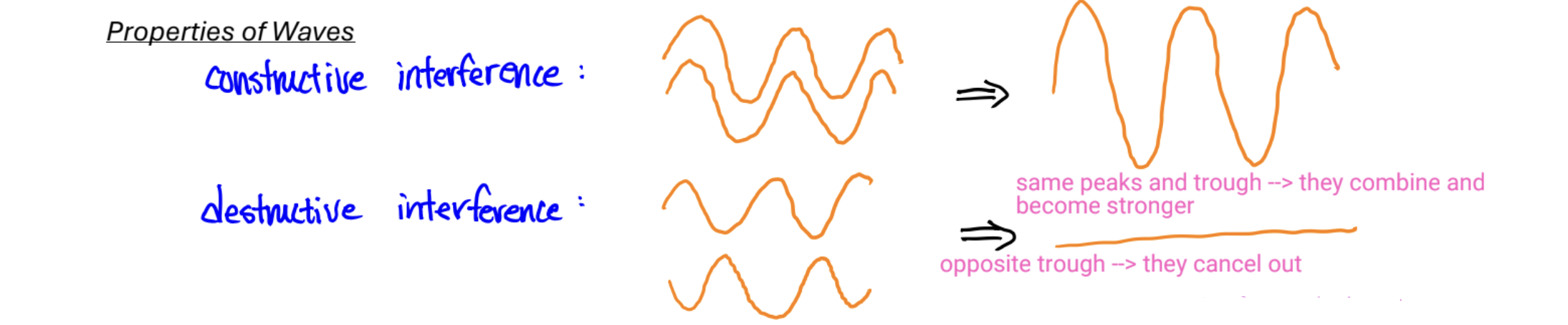
Properties of Waves
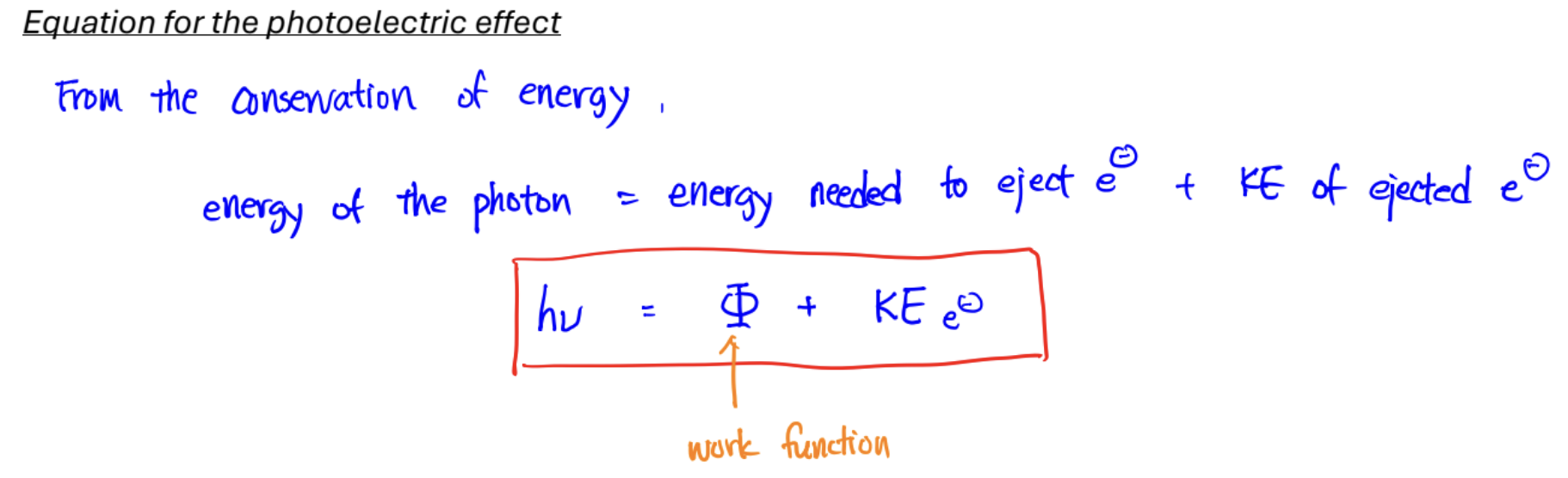
Photoelectric Effect
Experiment: shine light onto a metal plate; electrons bounce off
Einstein’s Explanation:
light exists as packets of energy, aka photons
the energy of a photon proportional to its freq: E = h*ν (ν is freq)
each photon can eject an e- if it has enough energy to break bond b/w metal and e-
leftover energy becomes KE of e- (@ threshold freq, it has just enough energy to break, KE = 0)
Significance: discovery of photons
Experimental observations:
higher intensity of light causes → more photons per sec → more e- ejected, but the KE of the ejected e- stays the same because energy of photon (E = h*freq) stays the same
There is a threshold frequency for different metals. Below the threshold frequency, photons don’t have enough energy to ejected e-
Increasing the frequency of the light → photons have more energy → increases KE of the ejected e-
de Broglie wavelength (to find wavelength for PARTICLE, not photon)
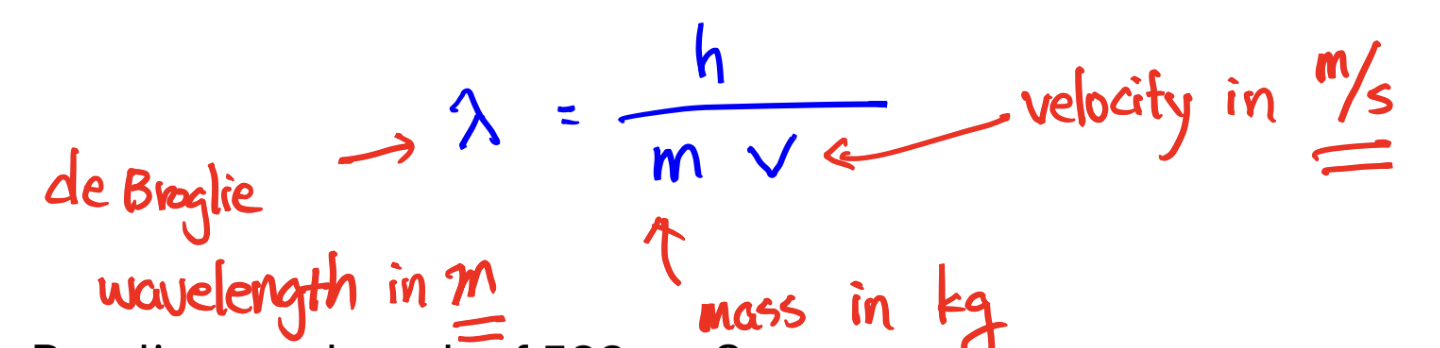
Two-Slit Experiment
Experiment: light shone on a screen with two slits.
if e- behaves like particle, no interference and light goes straight through to create two solid lines corresponding to the slits
BUT multiple lines were created
This suggested that, instead of behaving like particles, e- behaves like waves and that resulted in the interference pattern (distance b/w bright spots related to wavelength)
bright spot: constructive interference
dark spot: destructive interference
Significance: e- has wave-like behavior
Composition of Atoms
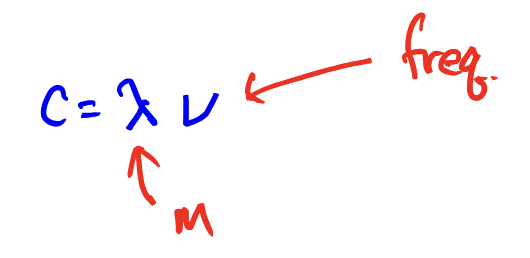
Electromagnetic Radiation: equation for calculating the wavelength of a PHOTON from its frequency
Electromagnetic Radiation: calculating the energy of a PHOTON from its frequency or wavelength
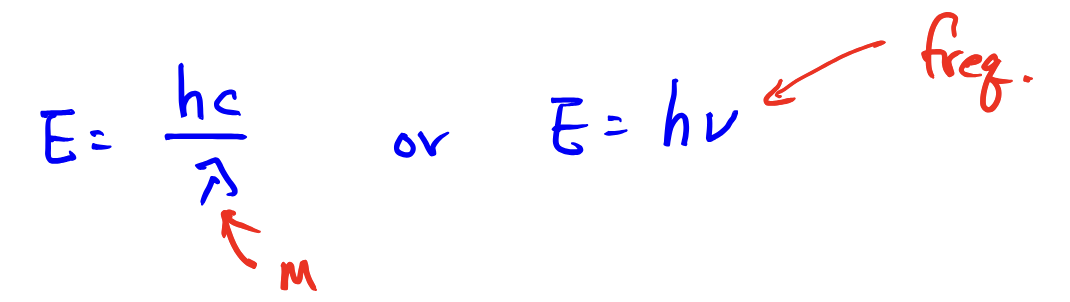
Absorption and Emission of Photons: definitions and equation
Absorption: when an e- absorbs a photon, it will jump to a higher level (only if the energy of the photon matches the energy difference)
Emission: when an e- drops to a lower level, it releases a photon whose energy is the differenc
Equation: for both,
E photon = |delta E| = |E of final - E of initial|
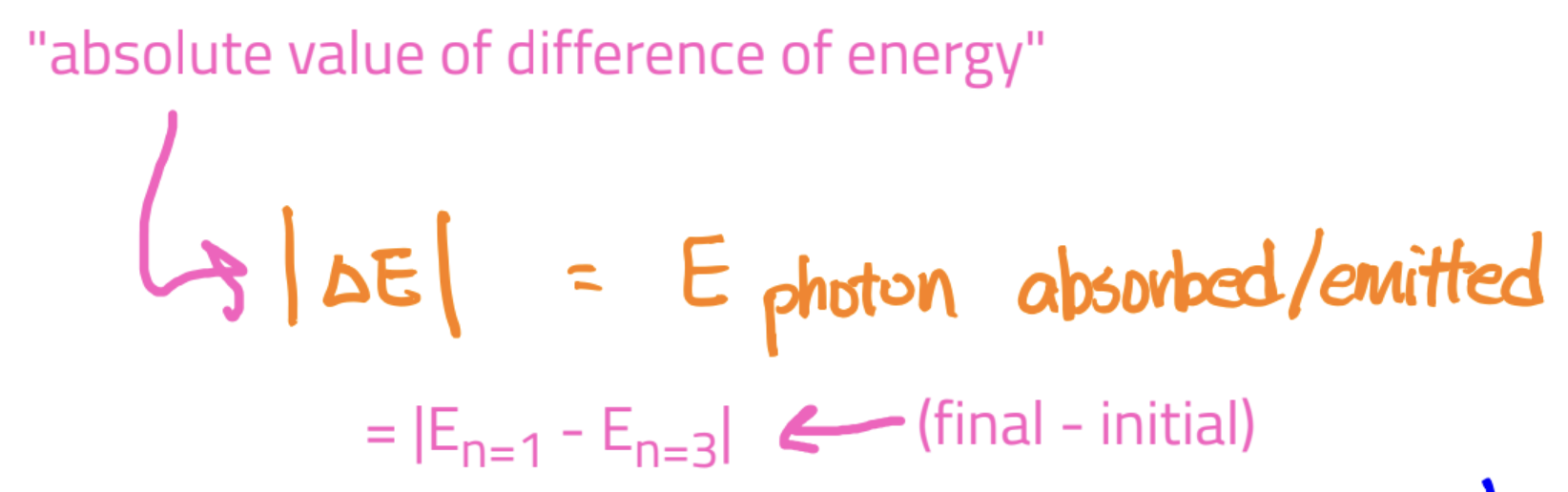
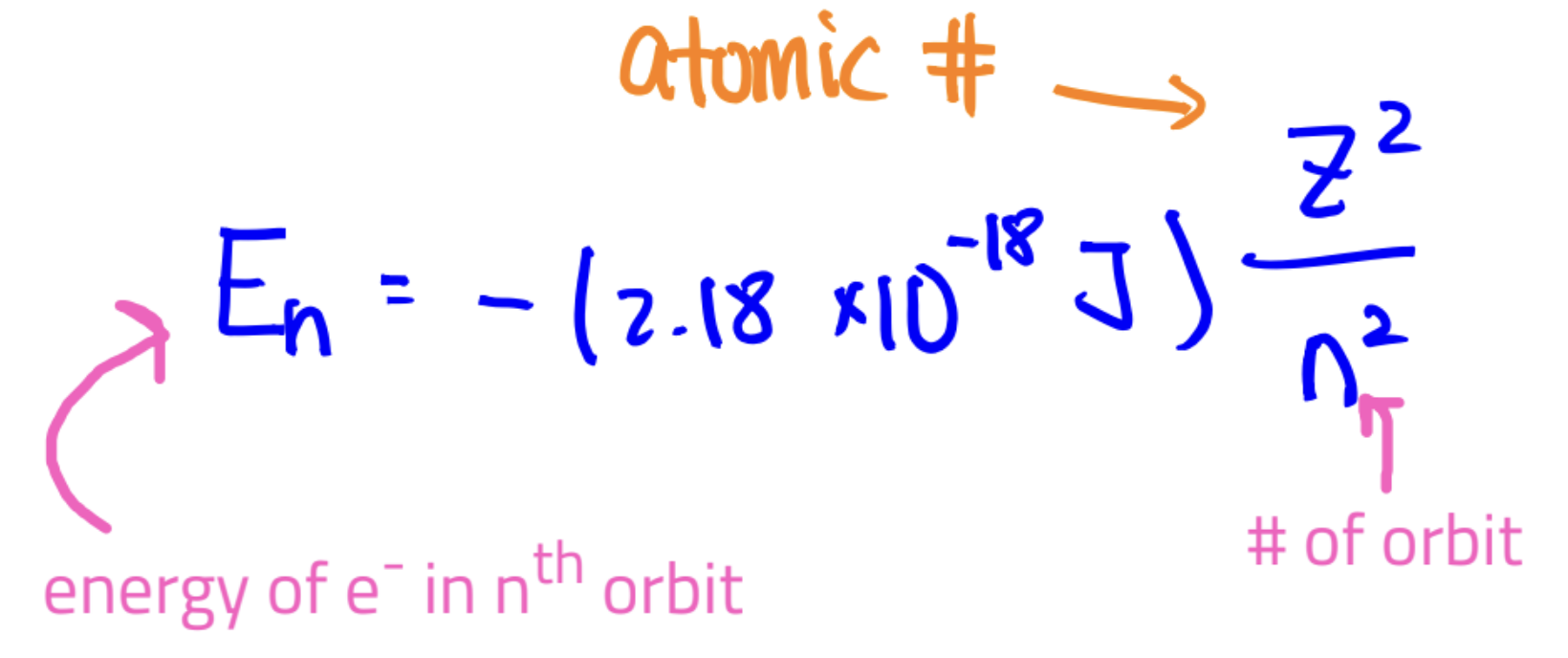
En for Bohr atom question
En for particle-in-a-box question
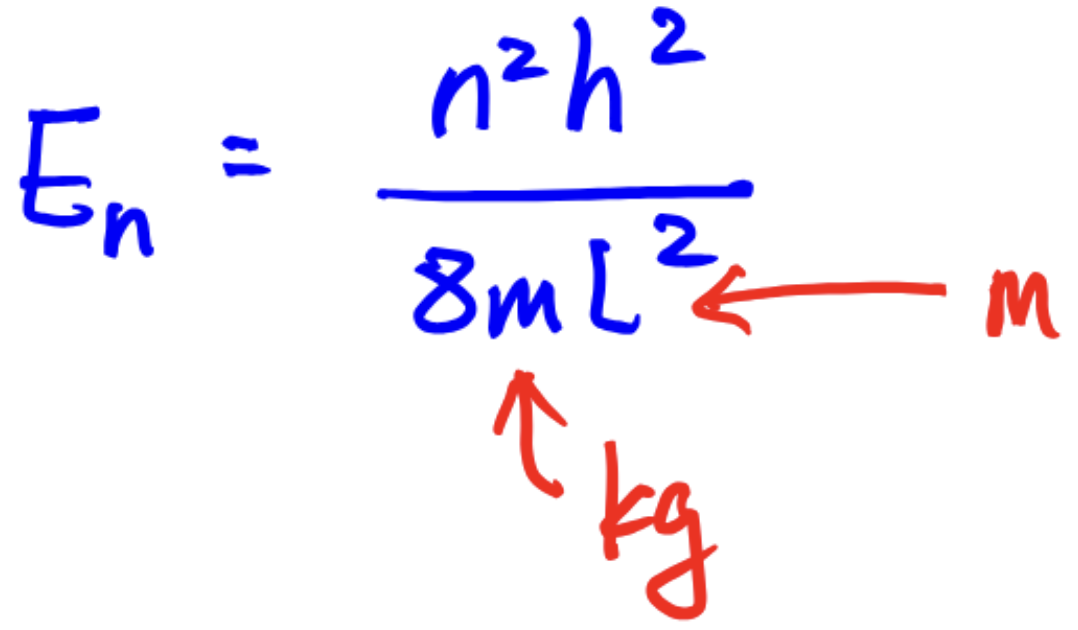
Wavefunction
a mathematical equation that describes the amplitude of a wave at different locations;
it has no physical meaning, but (wavefunction)² gives the probability of finding e-
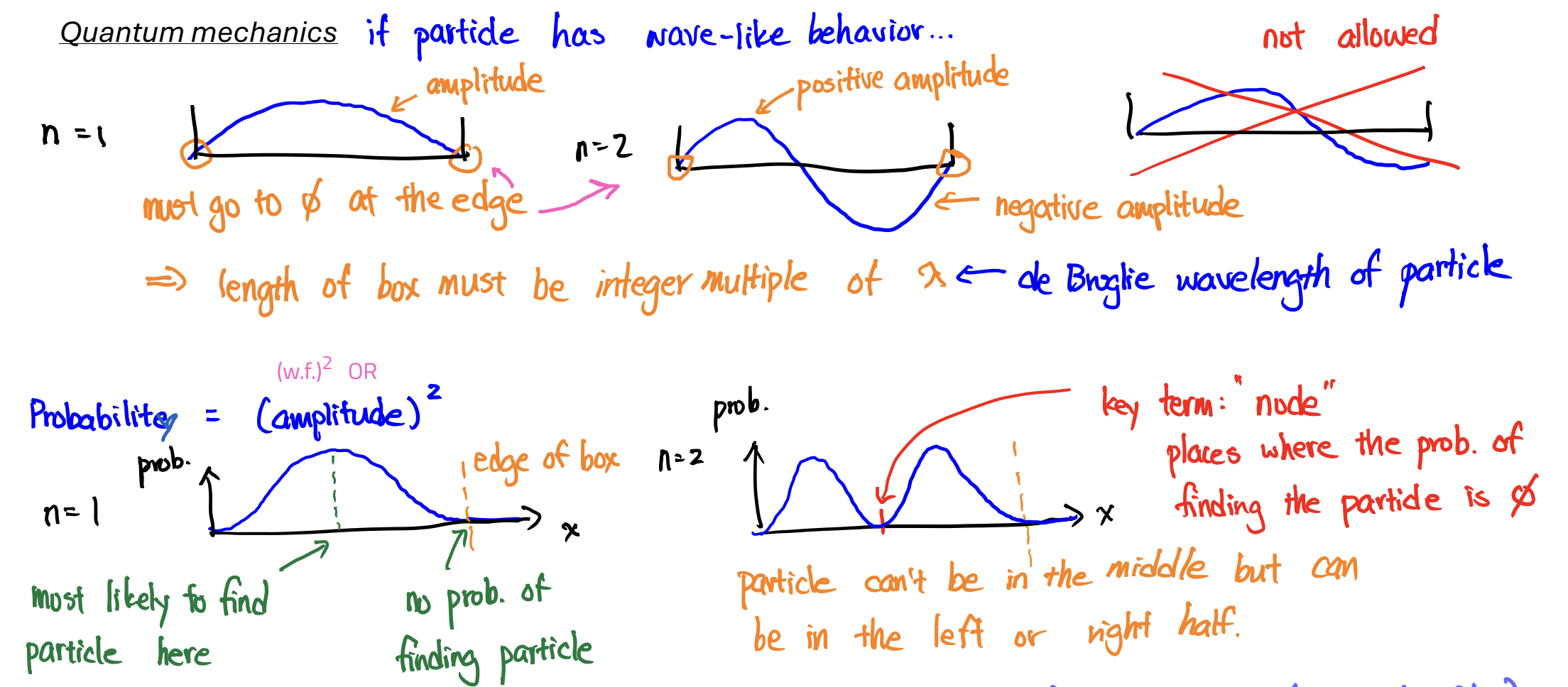
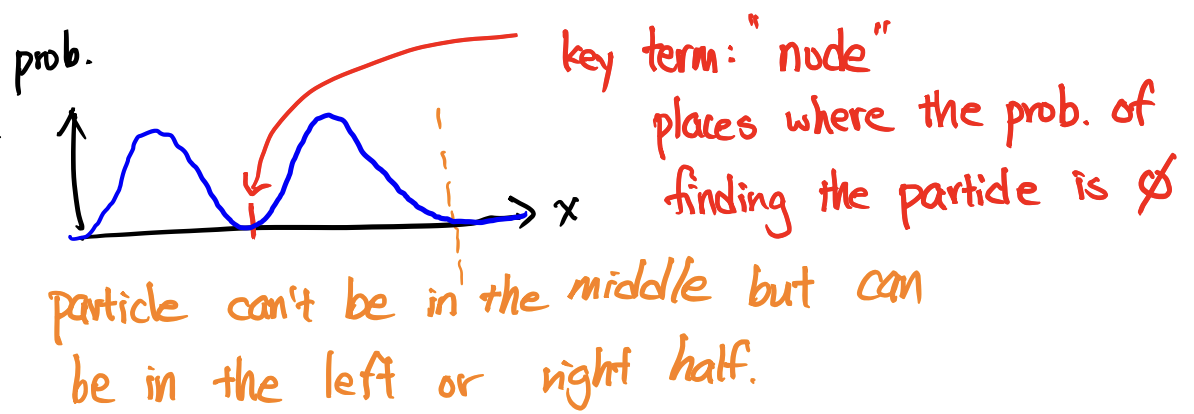
Node
probability of finding the particle is 0
n (shell)
Name: Principal quantum number
Value: 1, 2, 3,…
Determines: size
Rules: smallest allowed value is 1
As 𝑛 increases, the probability of finding the electron farther away from the nucleus increases.
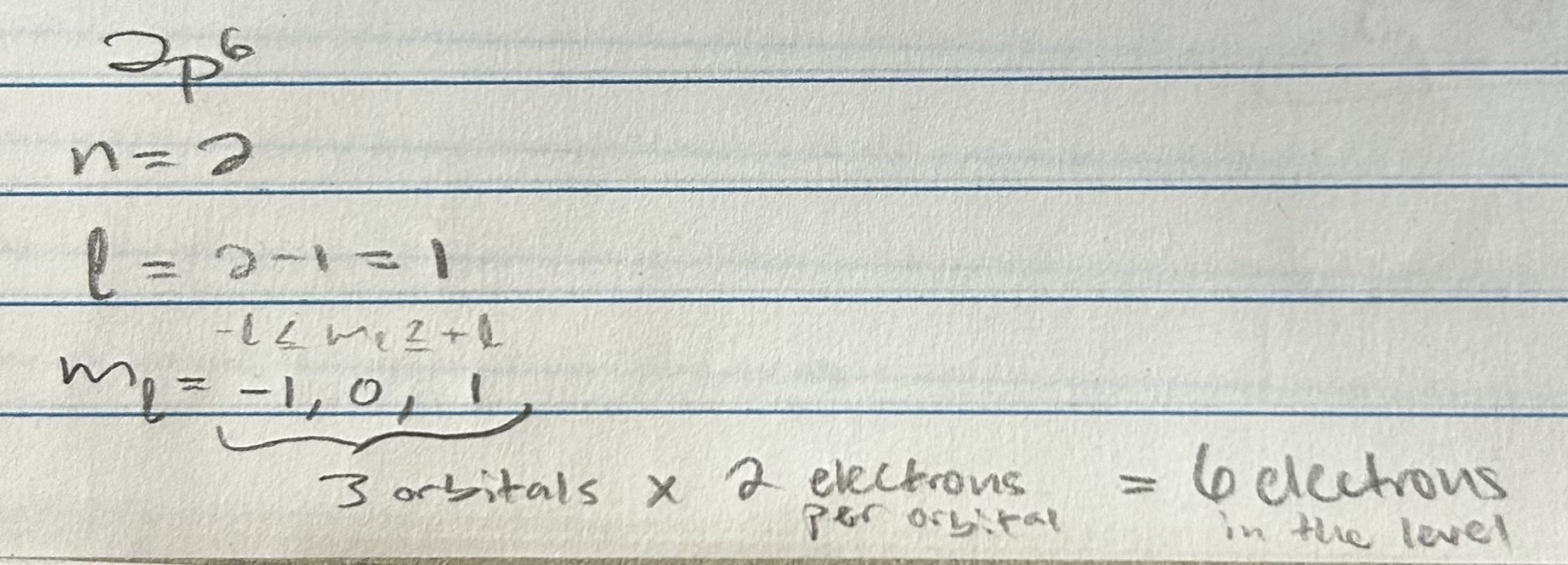
L (subshell)
Name: Angular momentum quantum number
Value: n - 1
Determines: shape
Rules: smallest allowed value is 1; largest, n-1
mL (orbital)
Name: Magnetic quantum number
Value: -L ≤ ml ≤ +L (the end points and what falls in the range)
Determines: orientation
Rules: allowed values depend on L
mS (spin)
Name: Spin quantum number
Value: +1/2, -1/2
Determines: spin of e-
How many subshells and orbitals are in the n = 3 shell?
(note: doesn’t specify L)
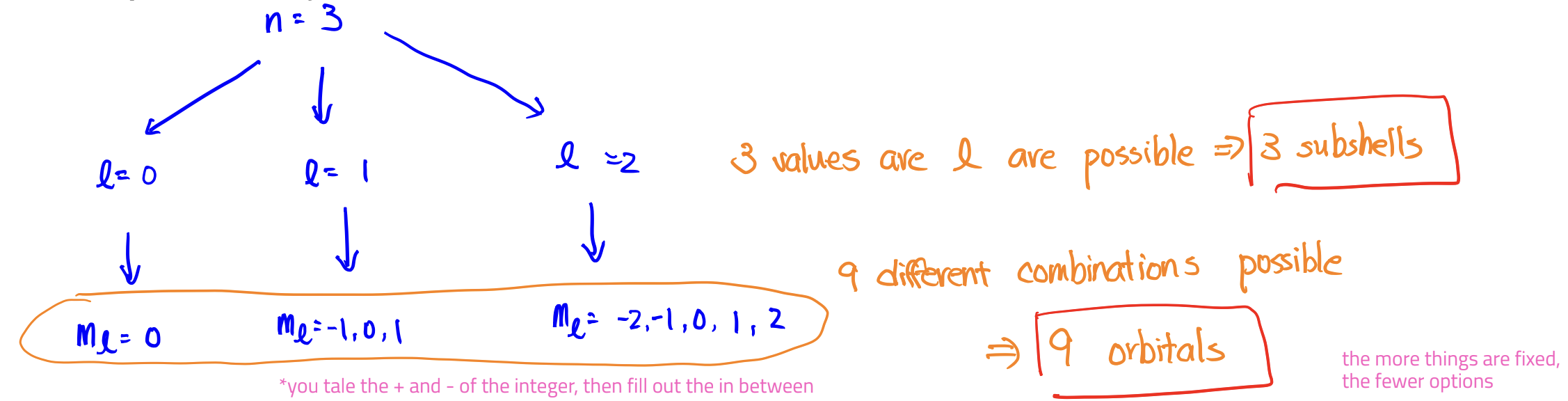
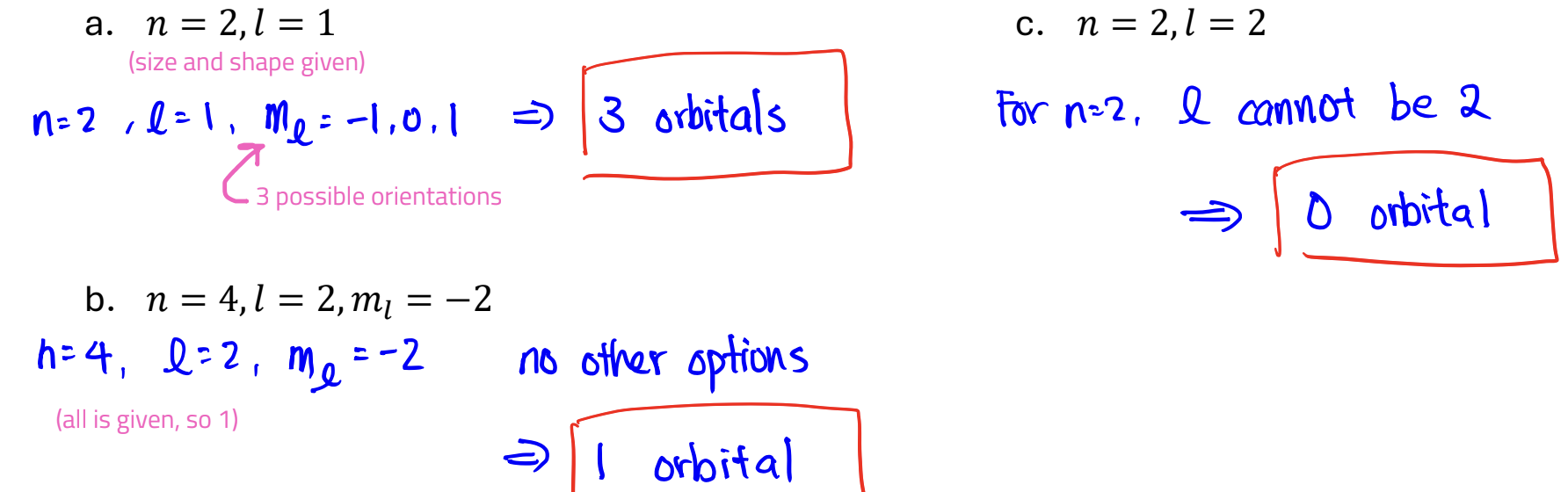
How many orbitals can have the following quantum numbers in an atom?
a. n = 2, l = 1
b. n = 4,l = 2, ml = −2
c. n = 2,l = 2
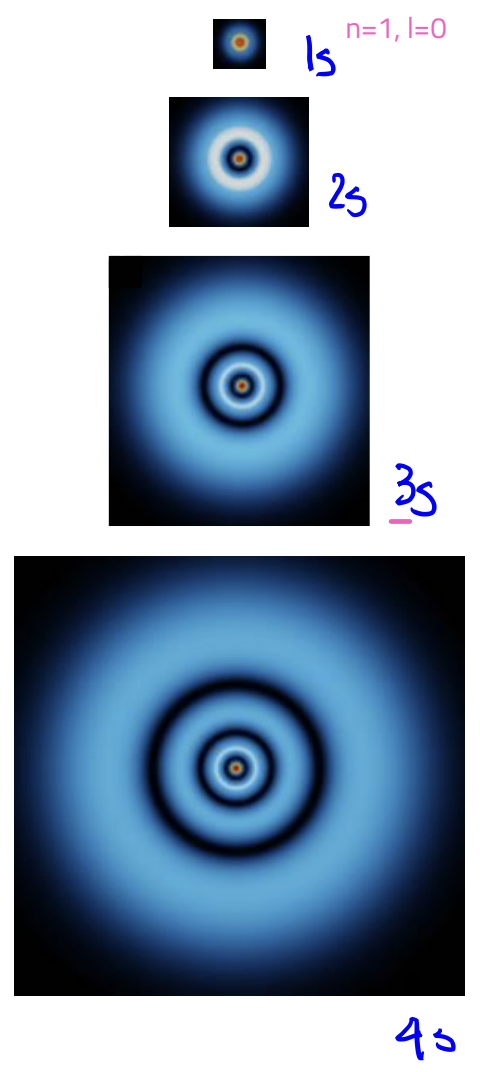
s-orbital
L = 0
angular nodes: 0
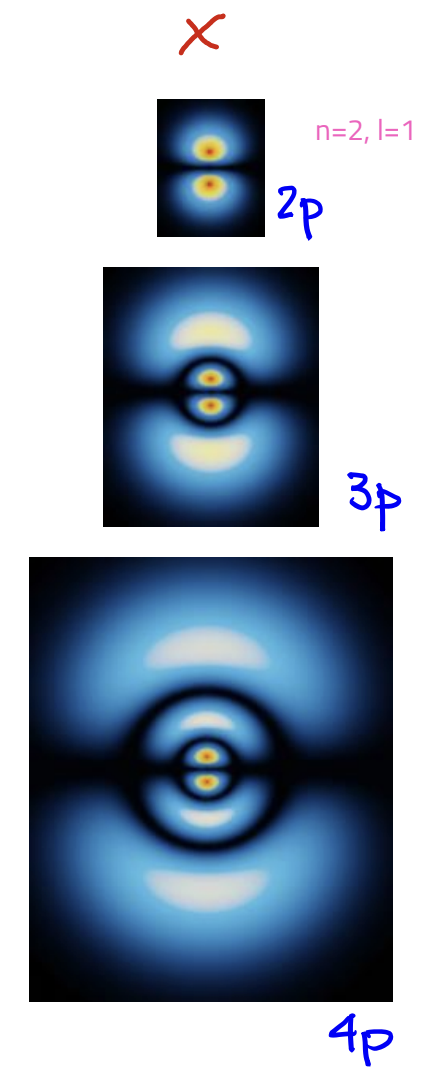
p-orbital
L = 1
angular nodes: 1
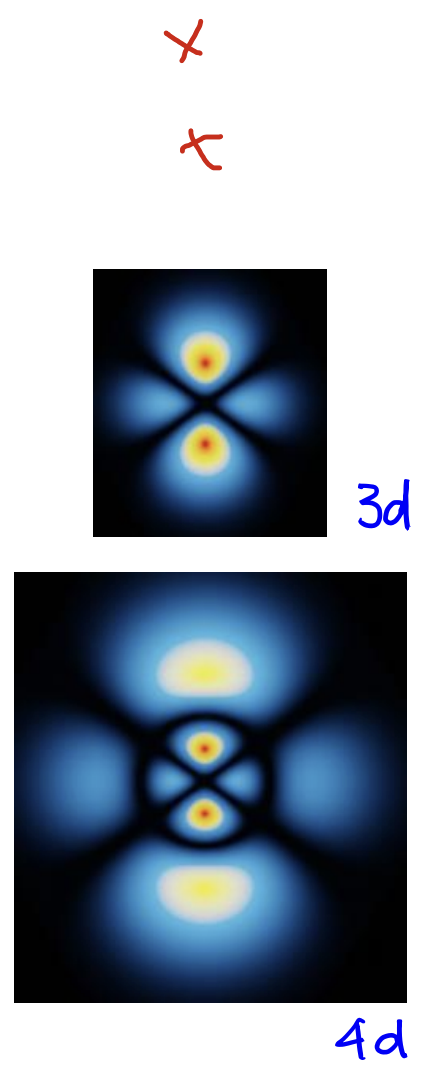
d-orbital
L = 2
angular nodes: 2
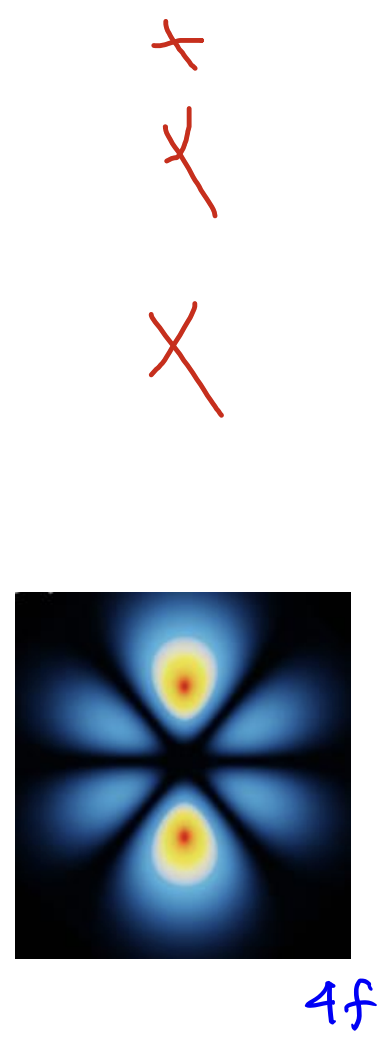
f-orbital
L = 3
angular nodes: 3
Effective Nuclear Charge
When there are multiple e-, e- found closer to the nucleus are less shielded
(if it’s closer, there are less other e- blocking; e-e repulsion is what causes the sheilding)
They will have a higher effective nuclear charge…
(it will feel more nuclear charge since it’s closer),
…making its energy lower (the stronger the attraction b/w the e- and nuc., the more stable and lower energy)
Energy Levels: Bohr vs Atomic Orbitals
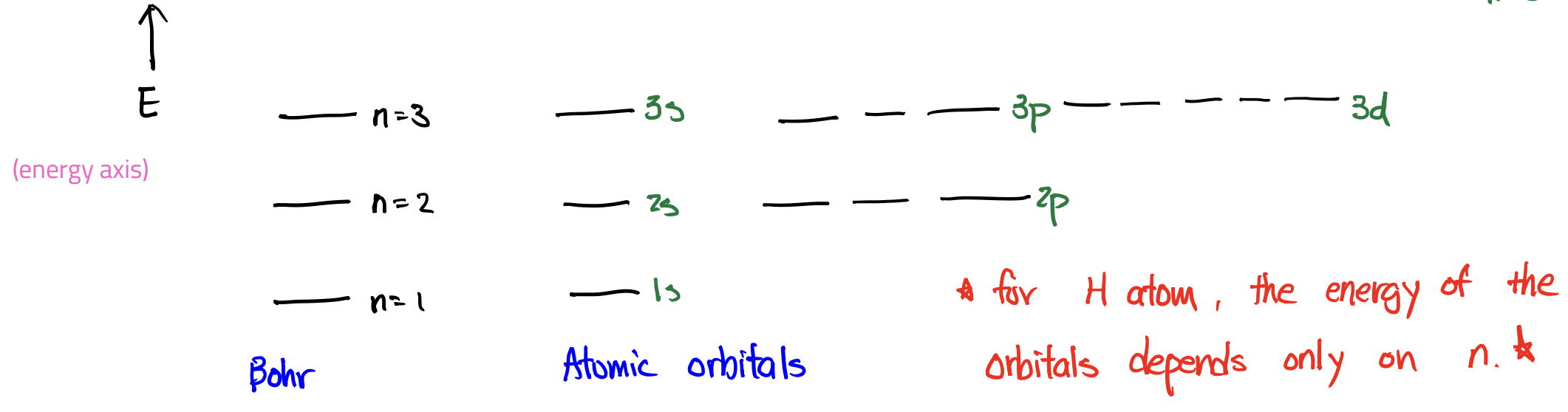
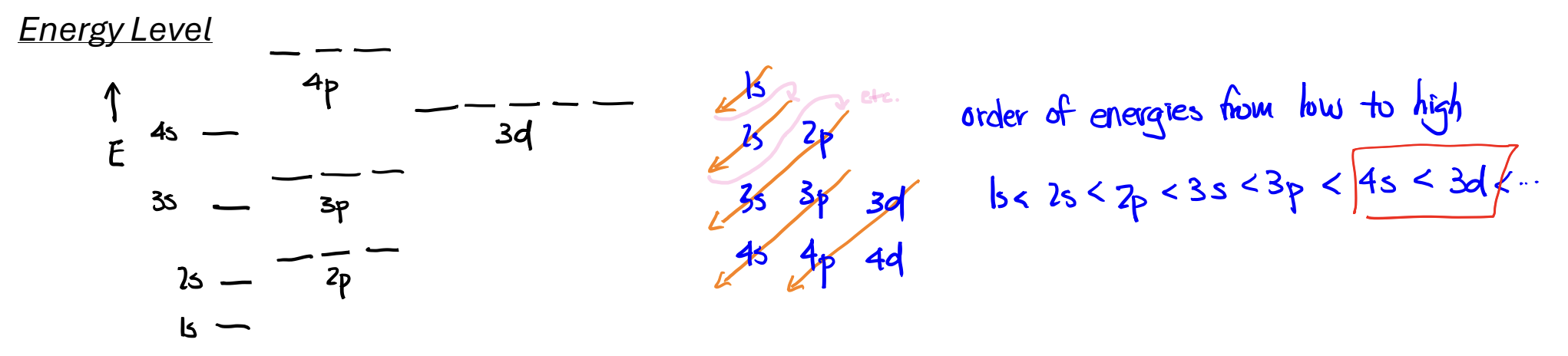
Energy Levels: Atomic Orbitals
General Rules for Writing Electron Configuration
Aufbau Principle: add Z electrons, one after another, to the orbitals with the lowest energy,
Pauli’s Exclusion: no more than two electrons in any one orbital.
Hund’s Rule: If more than one orbital in a subshell is available, add electrons with parallel spins to different orbitals of the subshell rather than pairing two electrons in one of the orbitals (if all full w/ 1, then ok to add a 2nd)
General Rules for Writing Electron Configuration
Add Z electrons, one after another, to the orbitals with the lowest energy (Aufbau), but with no more than two electrons in any one orbital (Pauli’s Exclusion).
If more than one orbital in a subshell is available, add electrons with parallel spins to different orbitals of the subshell rather than pairing two electrons in one of the orbitals (Hund).
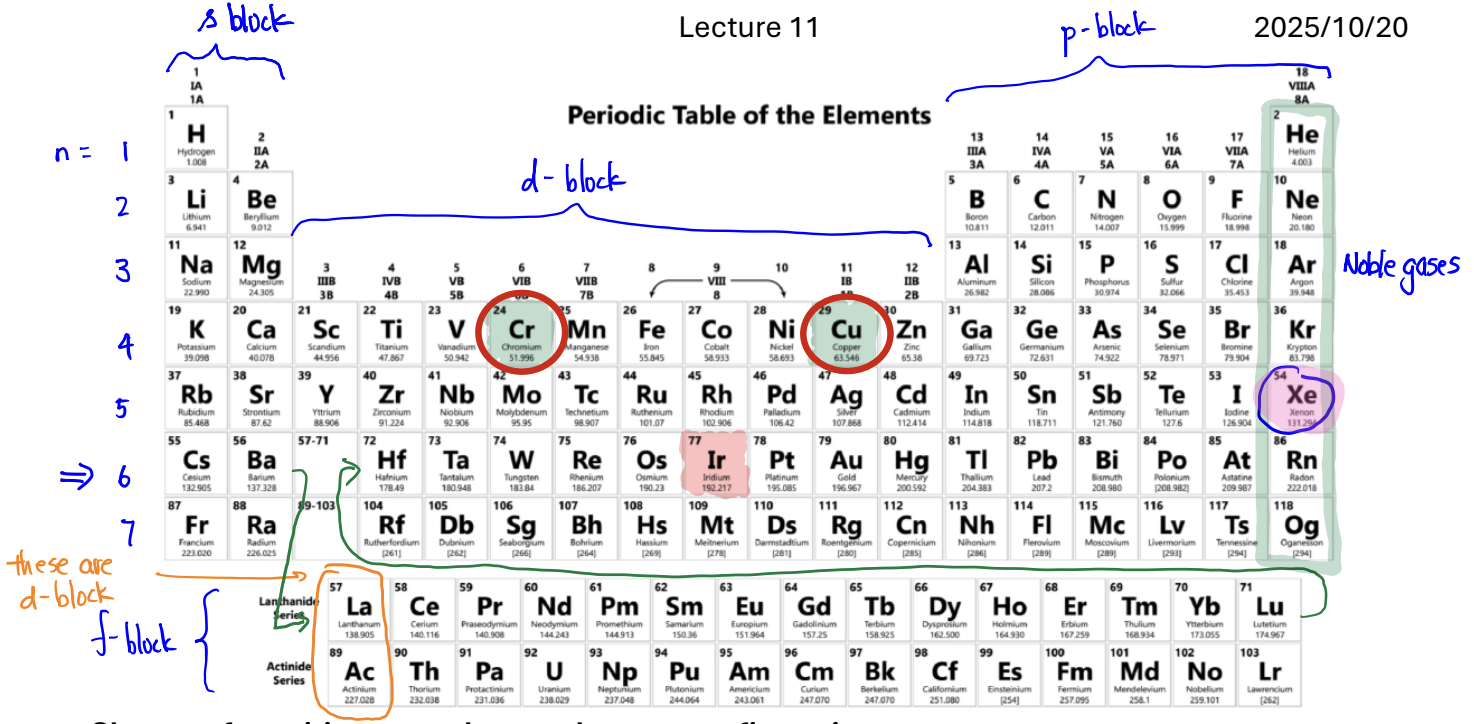
Shortcut for writing ground state electron configuration
Find the element on the period table.
Find the previous noble gas
Move toward the element of interest and fill as you go:
a. If you pass through s-block, fill ns orbital
b. If you pass through p-block, fill np orbital
c. If you pass through d-block, fill (n − 1)d orbital
d. If you pass through f-block, fill (n − 2)f orbital
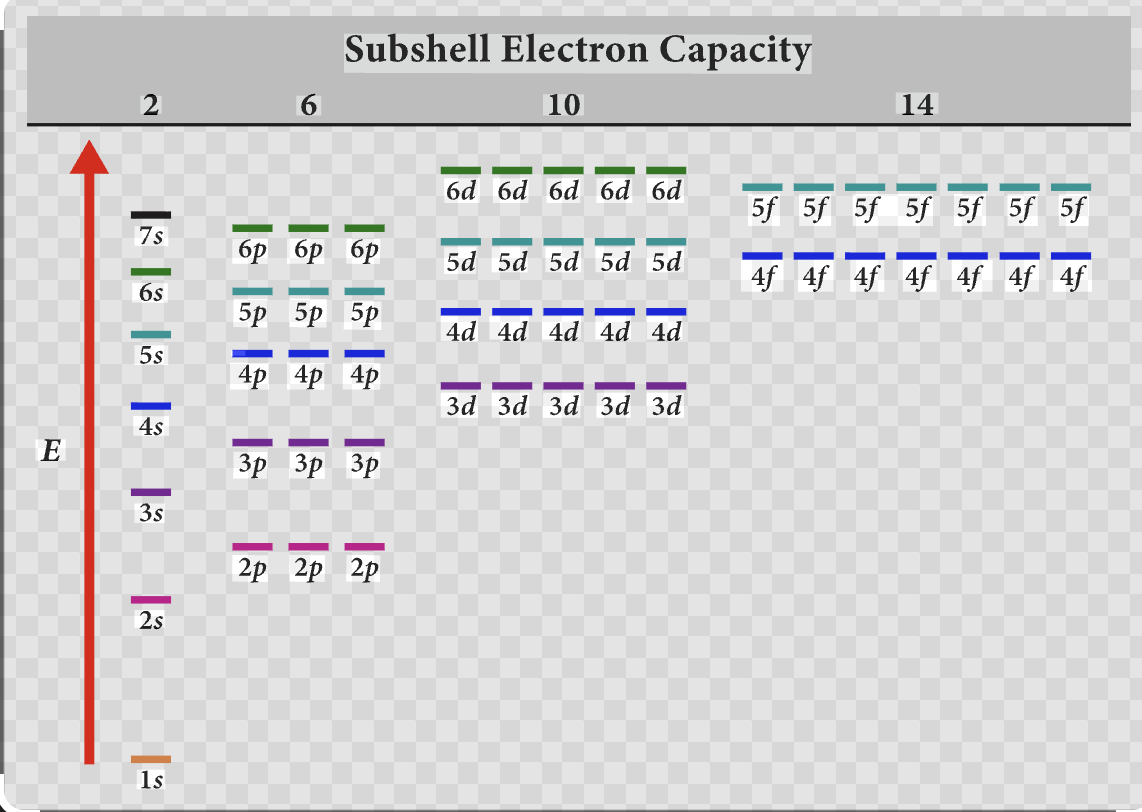
Subshell Electron Capacity
Two Exceptions of writing ground state e- configuration
Chromium (Cr) and Copper (Cu)
They’re written with one less e- in the s orbital—which is then added to the d orbital—because it would make them half filled; HALF-FILLED AND FILLED ARE MOST STABLE
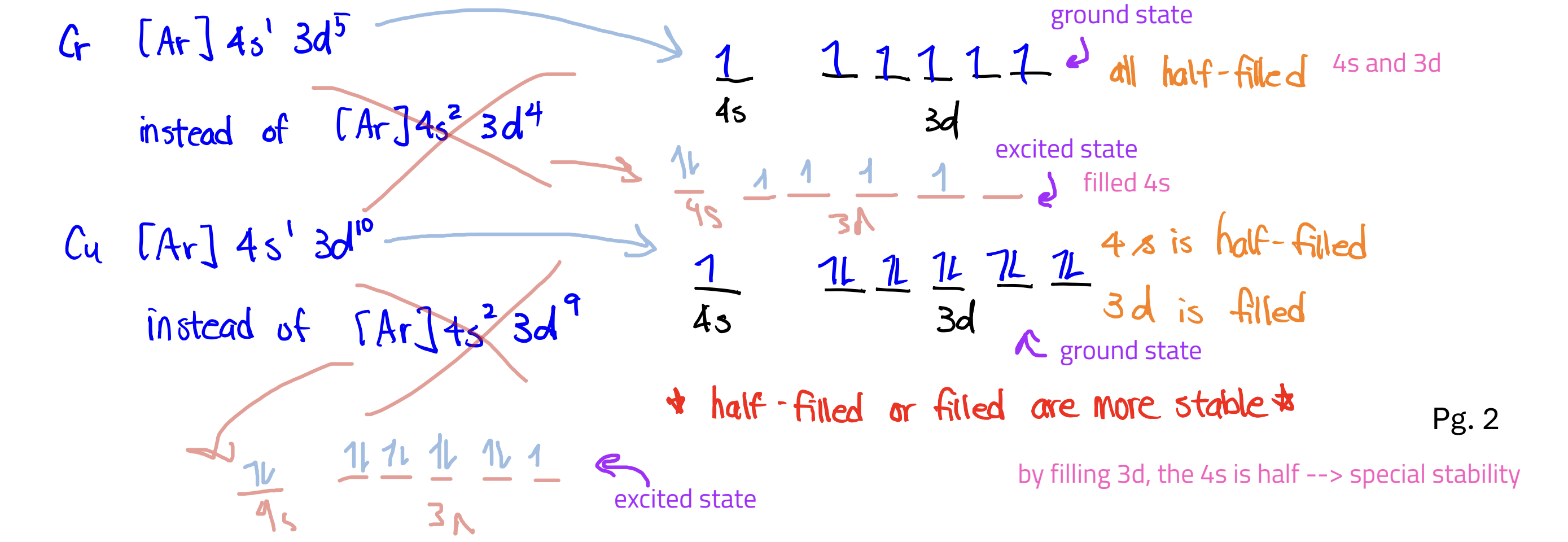
Ground-state electron configuration
allowed and lowest energy; follows all rules
Excited-state electron configuration
allowed but at higher energy (more unstable); violates Hund’s rule (parallel and separate subshells before pairing) or Aufbau’s principle (lowest energy first)
Forbidden electron configuration
not allowed; violates Pauli’s exclusion (no more than two electrons in any one orbital) or have nonexistent orbitals
Trends in a column are mainly explained by changes in…
n (size of the orbital)
going down group → n inc. → e- farther from nucleus
Trends in a row are mainly explained by changes in…
effective nuclear charge
going right in period → nuclear charge inc. (add proton), but e- also added
HOWEVER e- in same shell not as good at shielding → effective nuclear charge (Zeff) inc. going right
Atomic Radius
the avg. distance of e- from the nucleus
inc. going down group (down column → n inc → size of orbital inc. → bigger atom)
dec. going right of period (go right → Zeff inc. → stronger nuc—e- attraction (e- pulled closer) → smaller atom)
Electron Affinity
energy released when an e- is added
larger EA = easier to add e- (wants)
smaller EA - harder to add e- (doesn’t want)
EA dec. going down group (down group → e- added to higher n → less favorable to add e-)
EA inc. going right of period, but lots of exceptions (right of period → Zeff inc. → stronger nuc.—e- attraction → more favorable to add e-)
Ionization Energy
energy required to remove e-
1st ionization energy = removing 1st e-; 2nd is removing second
larger IE = harder to remove
smaller IE = easier to remove
1st IE dec. going down group (down group → inc. n → e- farther than nucleus → easier to remove)
1st IE inc. going right of period (going right → Zeff inc. → stronger nuc.—e- attraction → harder to remove
Ionization Energy Exceptions
Exception #1: 1st ionization energy of B is lower than that of Be, and first ionization energy of Al is lower than that of Mg
for Al and B, removing from p orbital; for B and Mg, removing from s orbital
Exception #2: First ionization energy of O is lower than that of N, and first ionization energy of S is lower than that of P
half-filled subshells more stable → harder to remove
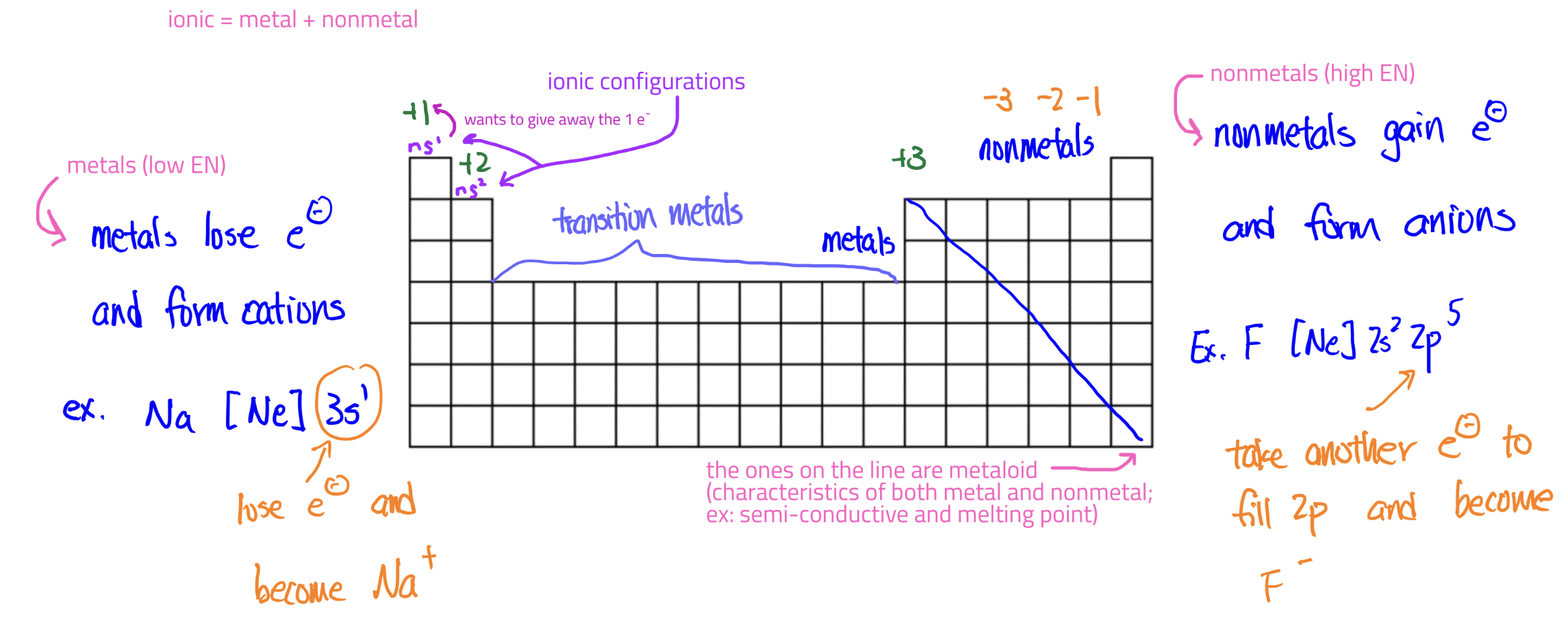
Valence Electons
electrons in the highest orbitals (highest n)
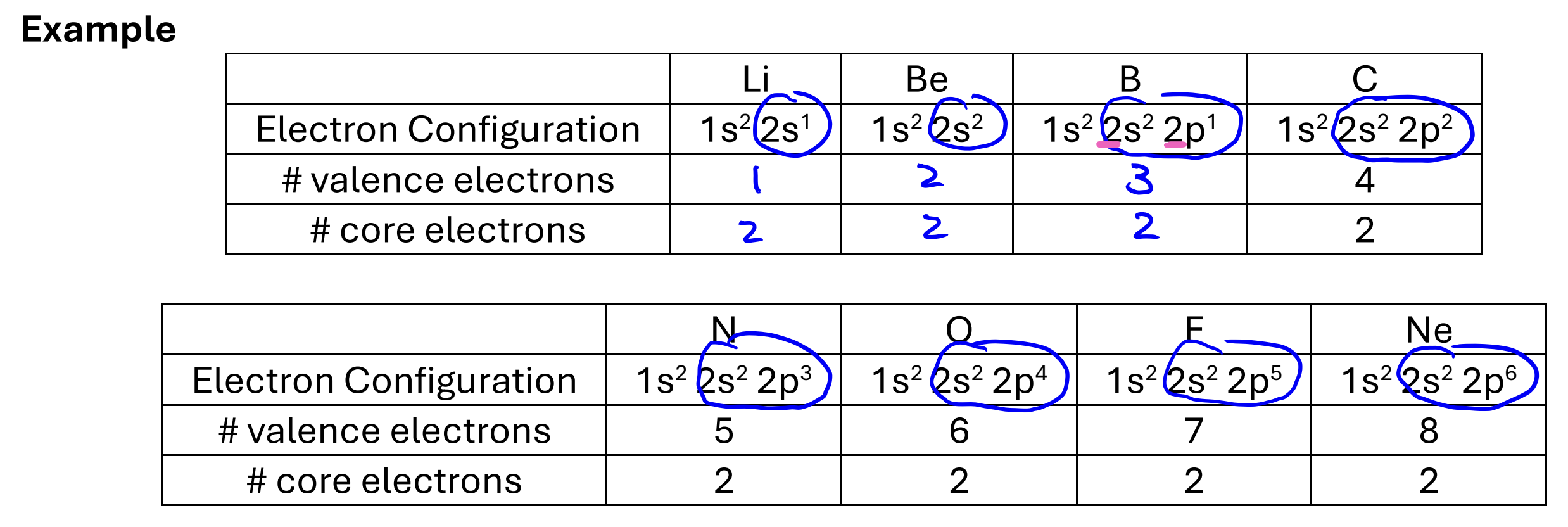
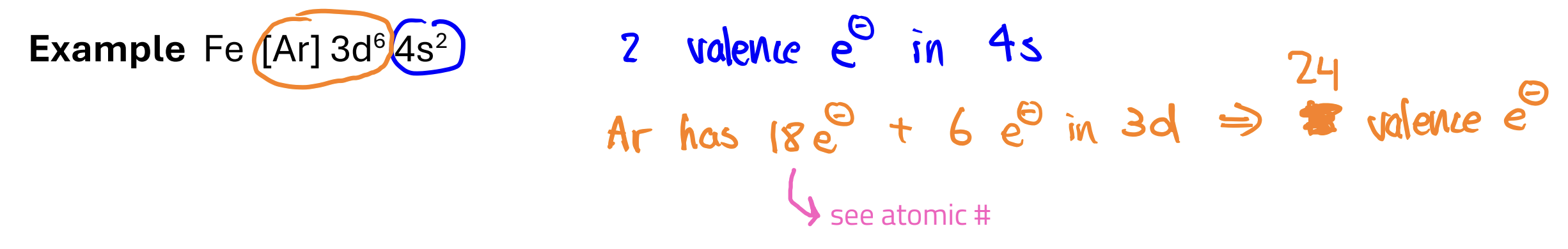
Core Electrons
all the other electrons not in the outermost orbital
Electronegativity
a scale indicating the electron-pulling power of an element (like a tug-of-war)
Larger differences in electronegativities means more unequal sharing of e-
dec. going down group
inc. going right period
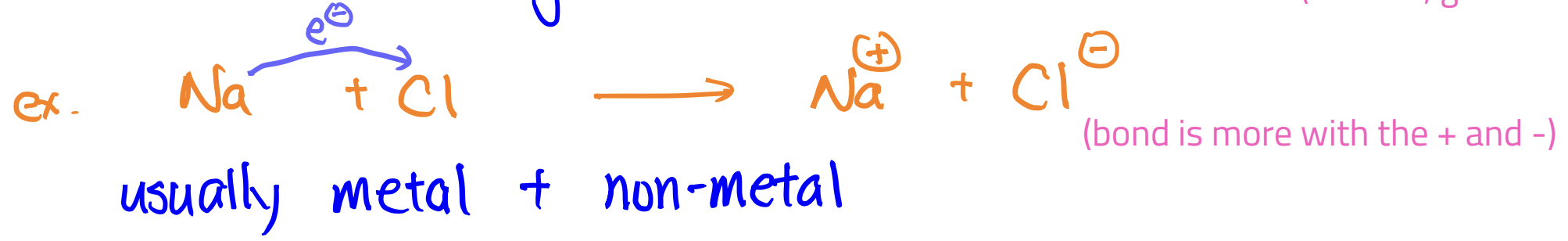
Ionic Compound
difference in EN is greater than 2.0 → e- is not shared (e- gets taken by one)
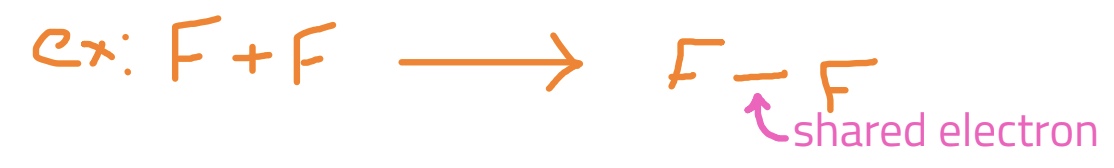
Covalent Compound
difference in EN is less than 2.0 → e- is shared
Nonmetals and Metals in Ionic Compounds
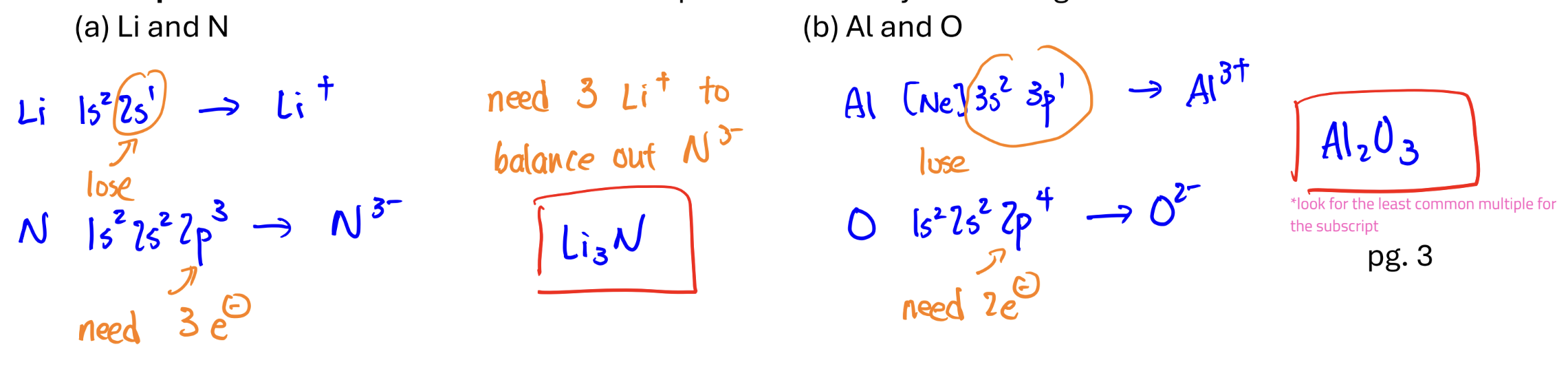
formula of an ionic compound
look at group number to see if it’ll take or get taken from; remember to balance out if necessary
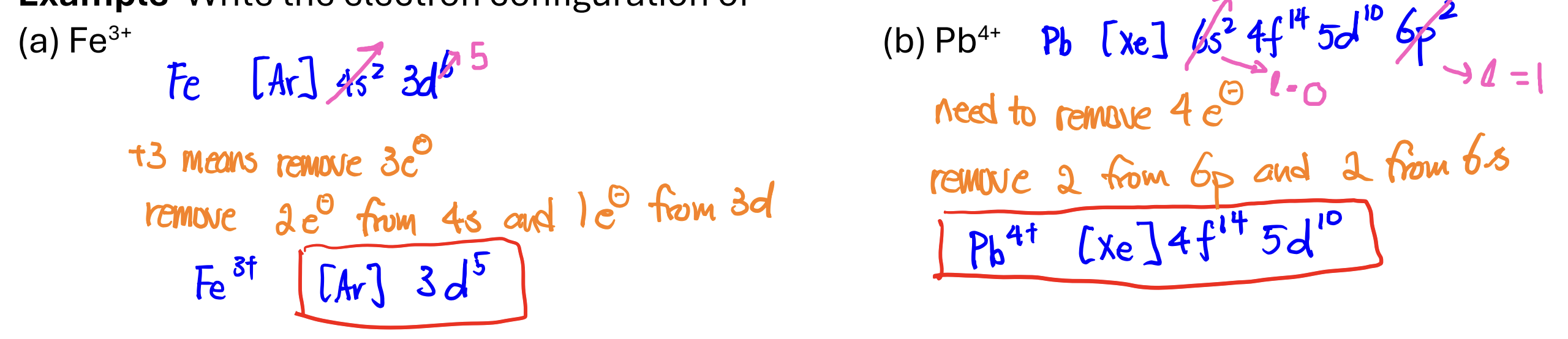
Electron Configuration of Transition Metal Cations
write the neutral electron configuration
remove e- from the highest n and highest L first; ex: remove from np, ns, (n-1)d
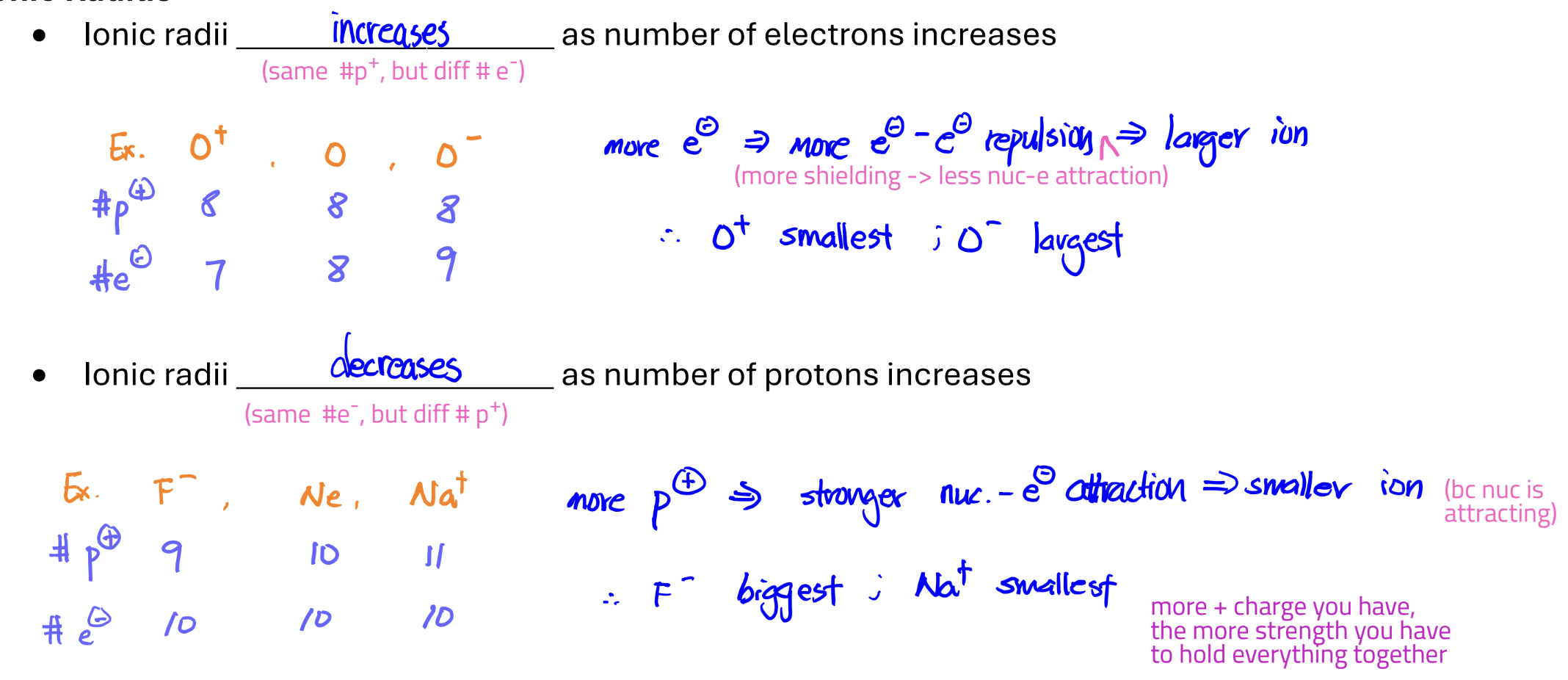
Ionic Radius inc./dec. when…
ionic radii inc. when # of e- inc.
ionic radii dec. when # of protons inc.
Lattice Energy
the attraction forces b/w cations and anions
can be thought of as the “bond strength” of ionic compound
stronger lattice energy → stronger attraction → more stable
bigger when charges are bigger or when ionic radii are smaller (when both charges and sizes are different, charge dominates)
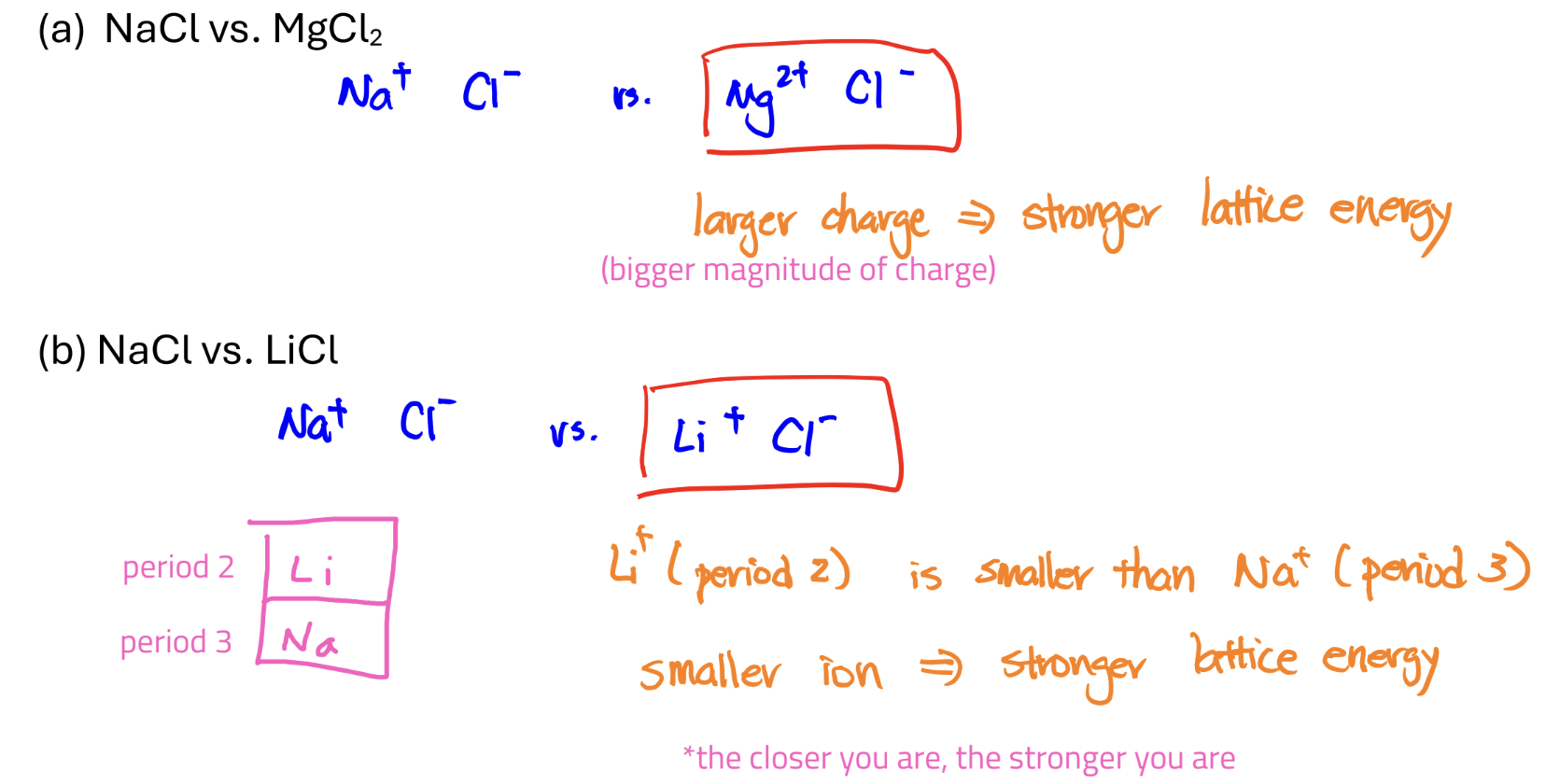
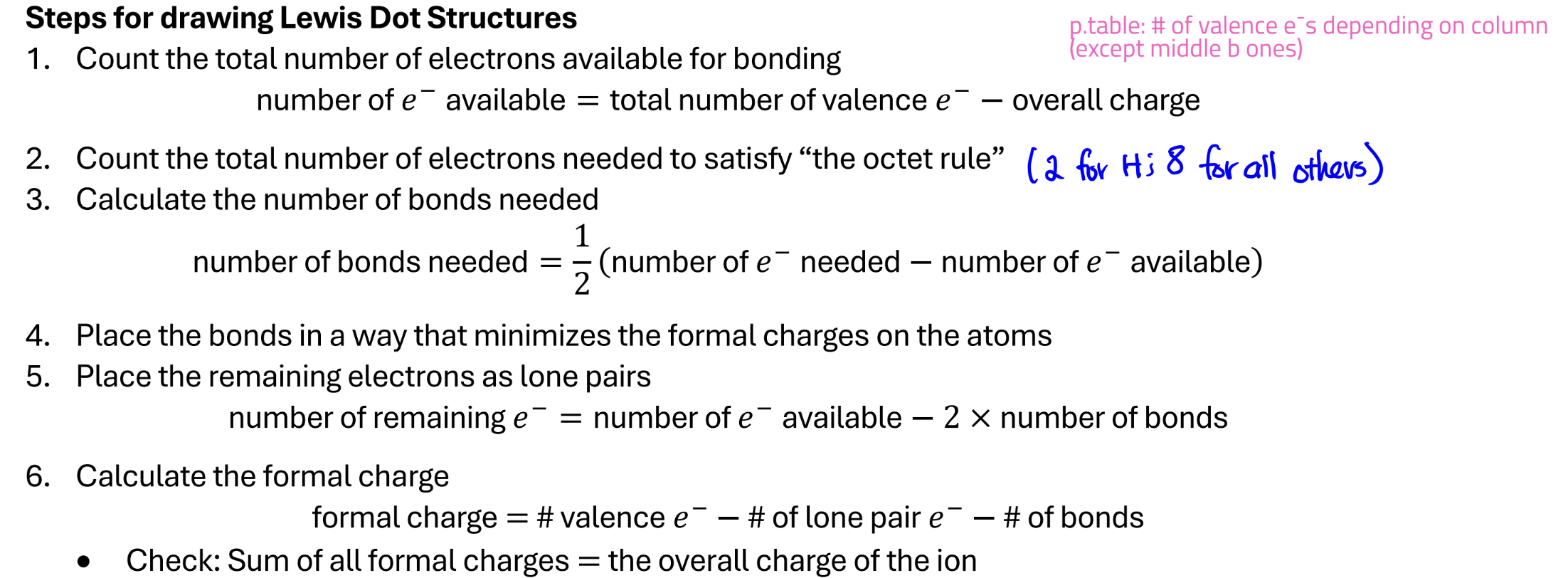
Lewis Dot Structure
helps us keep track of valence electrons
LDS of ionic compounds; try to satisfy octet rule
Steps for drawing Lewis Dot Structures
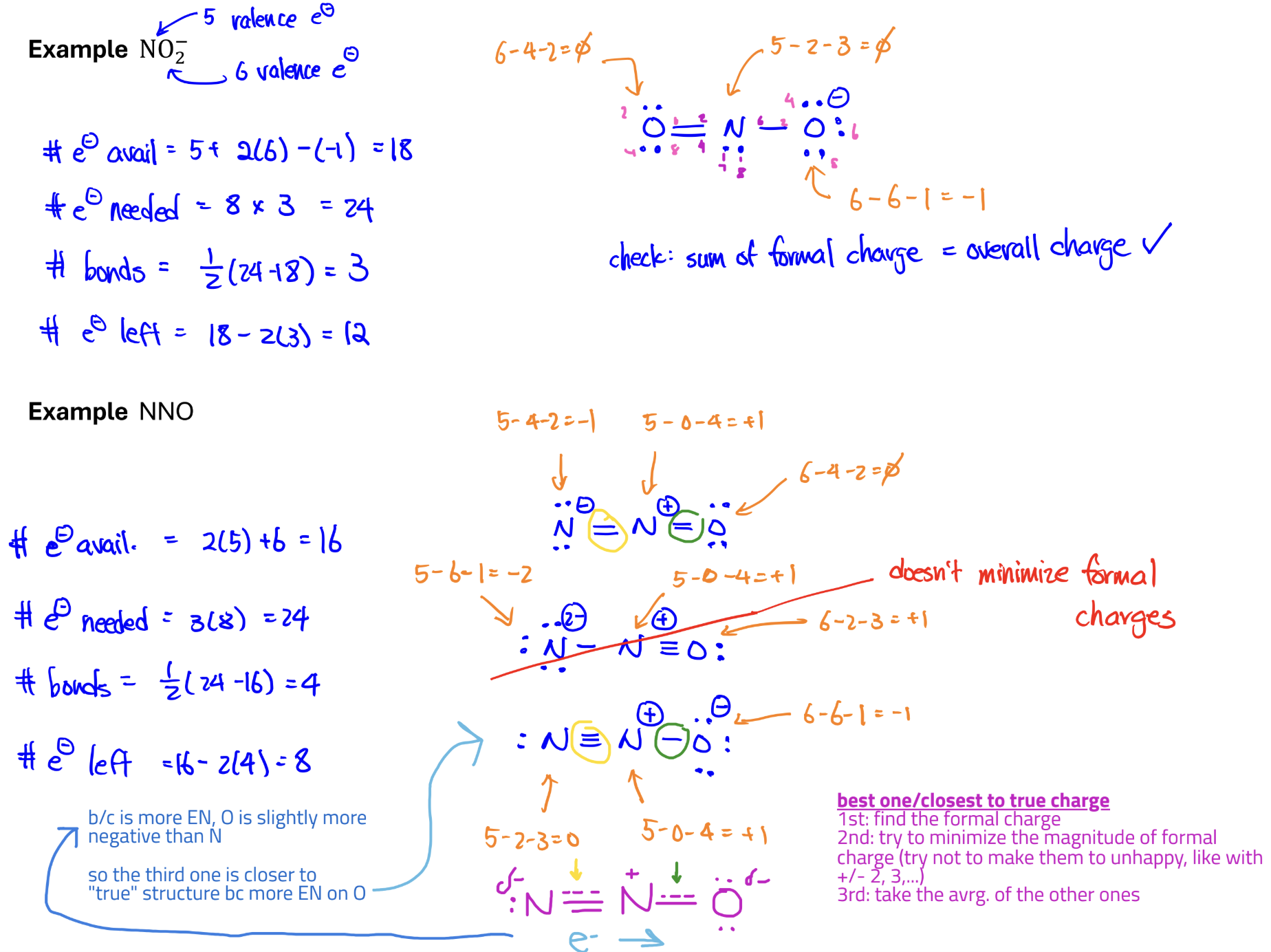
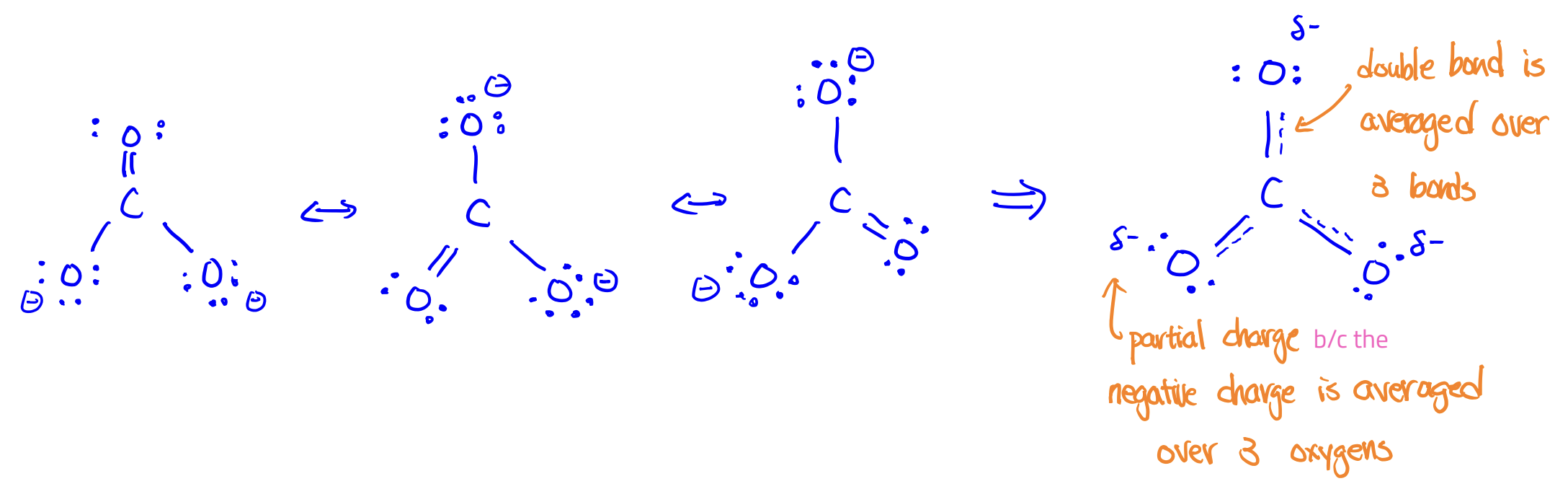
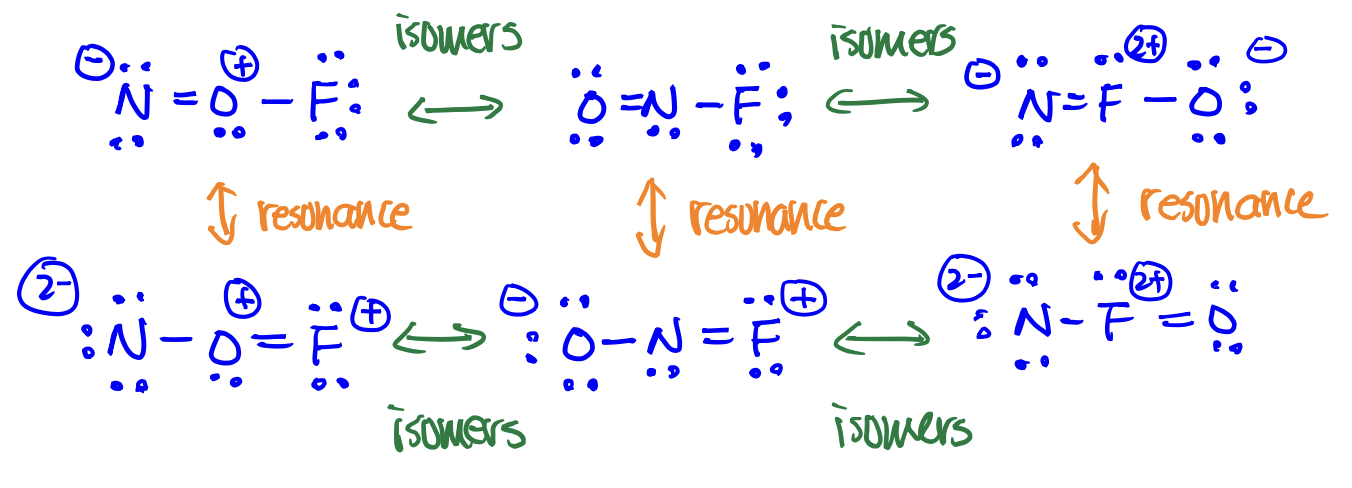
Resonance structures
structures with the same arrangement of atoms but with different arrangements of e-
Isomers
structures with different arrangement of atoms
*The true structure of a molecule is a blend of all the resonance structures
Lewis Dot Structure with Expanded Octet
For elements below period 2, you can expand octet to minimize formal charges.
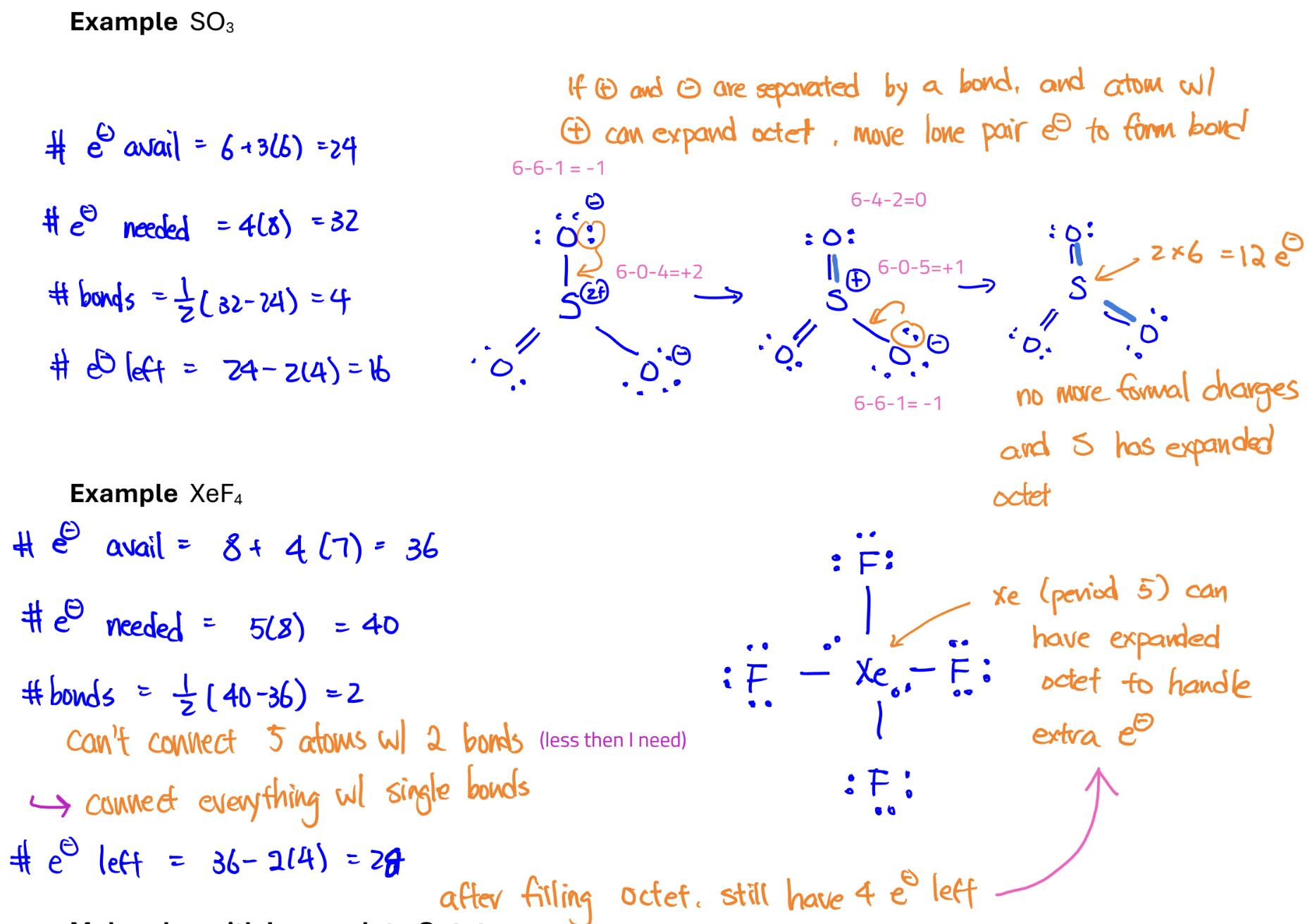
Molecules with Incomplete Octet
B and Al can form molecules with incomplete octet, usually of the form AX3
Radicals
molecules with unpaired e-
usually very reactive
Rules for drawing
Add an e- to the # of available electrons
Follow the typical rule for drawing a Lewis dot structure
Remove an e- from the atom with the most negative formal charge
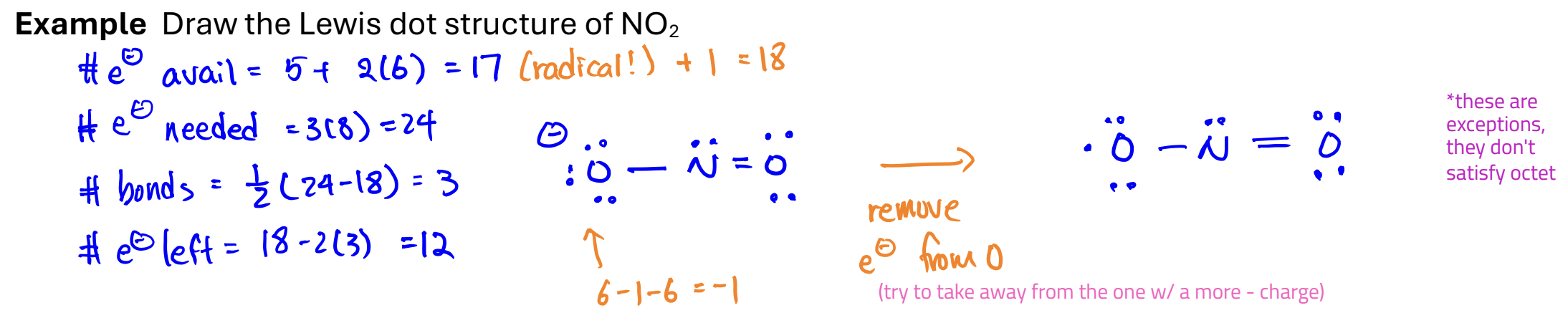
Internuclear Potential Energy Diagram
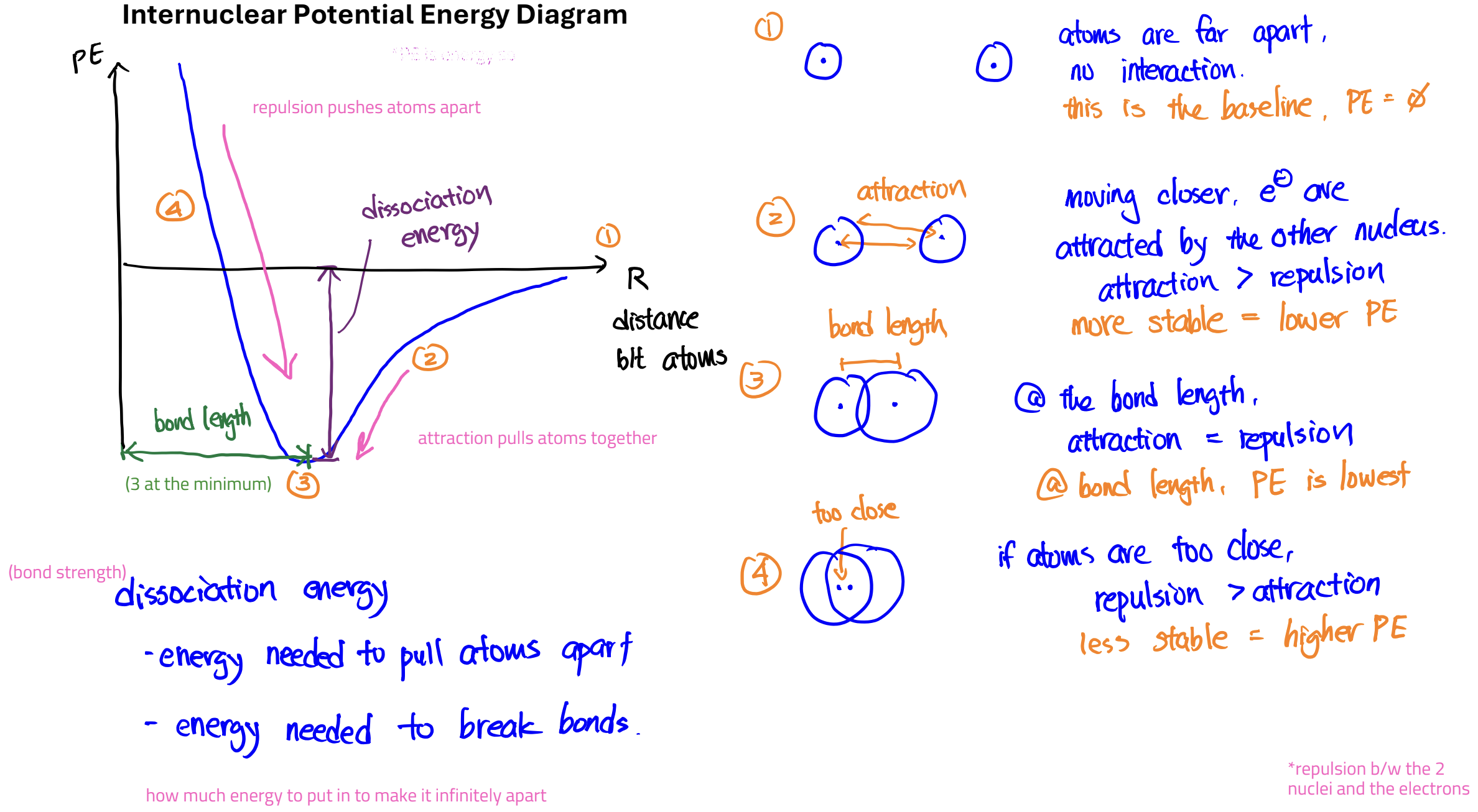
Larger bond dissociation energy (deeper well) means stronger bond (lower energy/more stable)
Relationship between Atomic Radii, Bond Length, and Bond Strength
Bigger atomic radius usually means longer bond length
Longer bond length usually means weaker bond
Multiple bonds: more bonds ➔ stronger bond ➔ shorter bond length
Polar covalent bond vs. nonpolar covalent bond
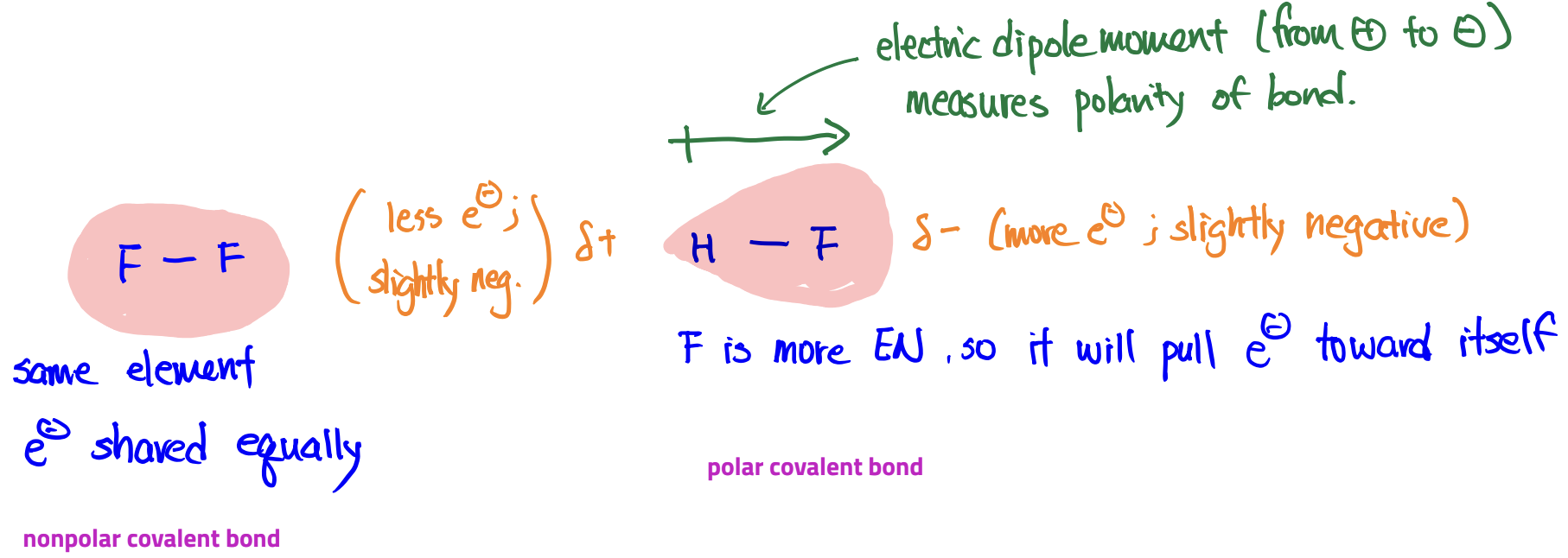
All covalent bonds between different elements are polar to some extent
polar due to difference in EN
Only covalent bonds between atoms of the same element are nonpolar (exceptions: C-H and Si-H)
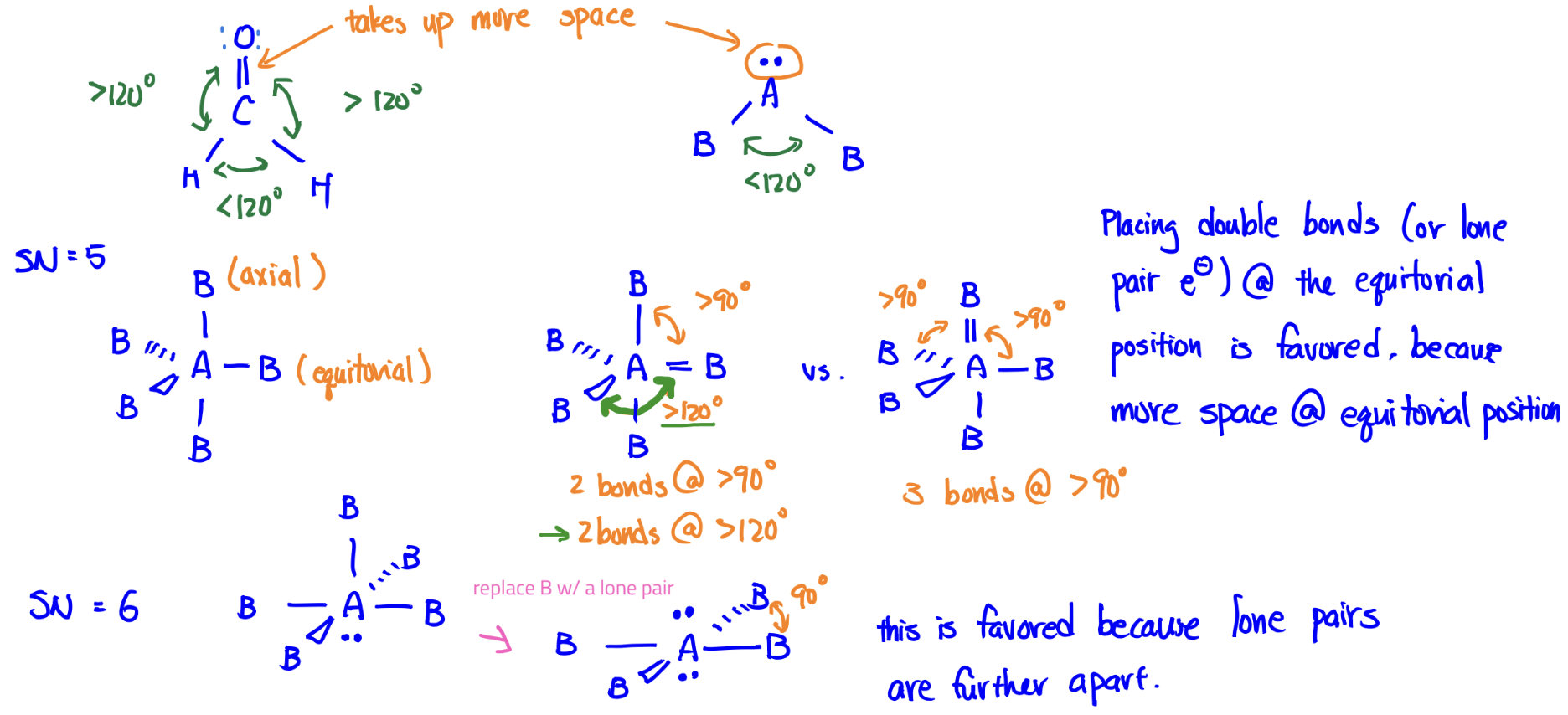
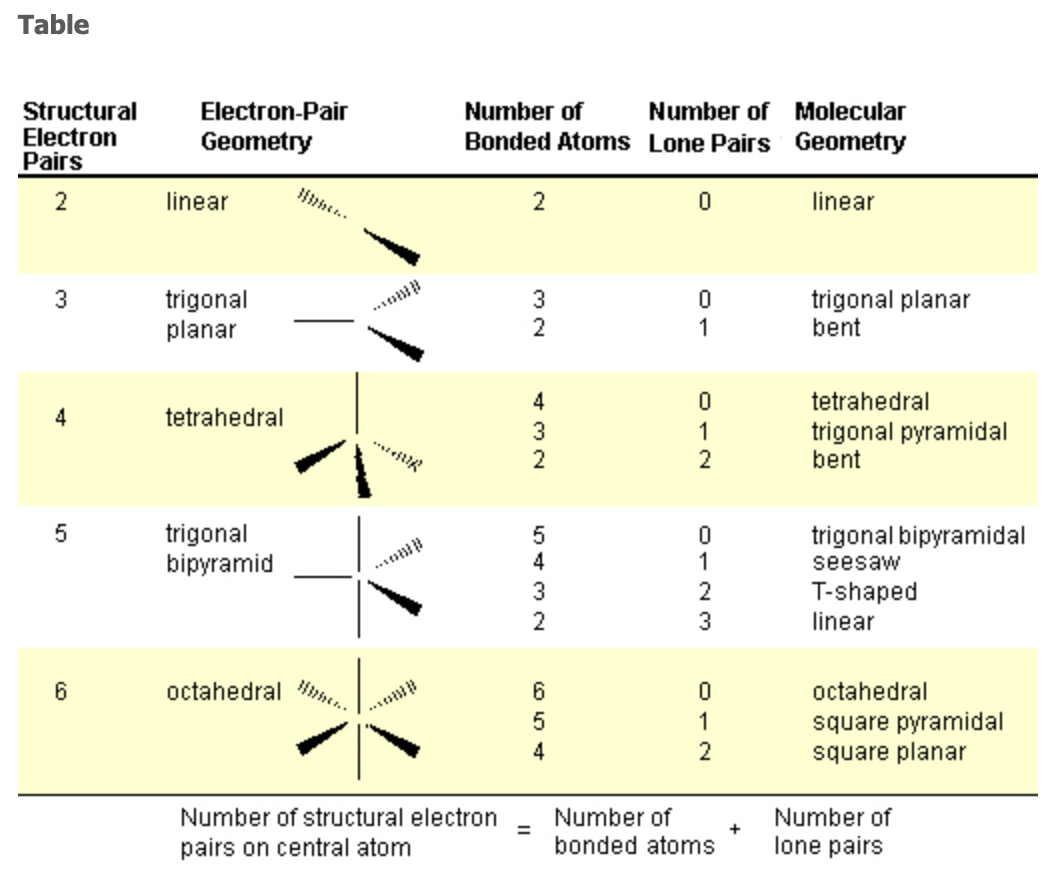
VSEPR Model
good at explaining shape; can help predict polarity
Bonds and lone pairs repel one another and, to minimize their repulsions, the bonds and lone pairs move as far apart as possible while maintaining the same distance from the central atom.
Steric Number = # bonds + # lone pairs around central atom
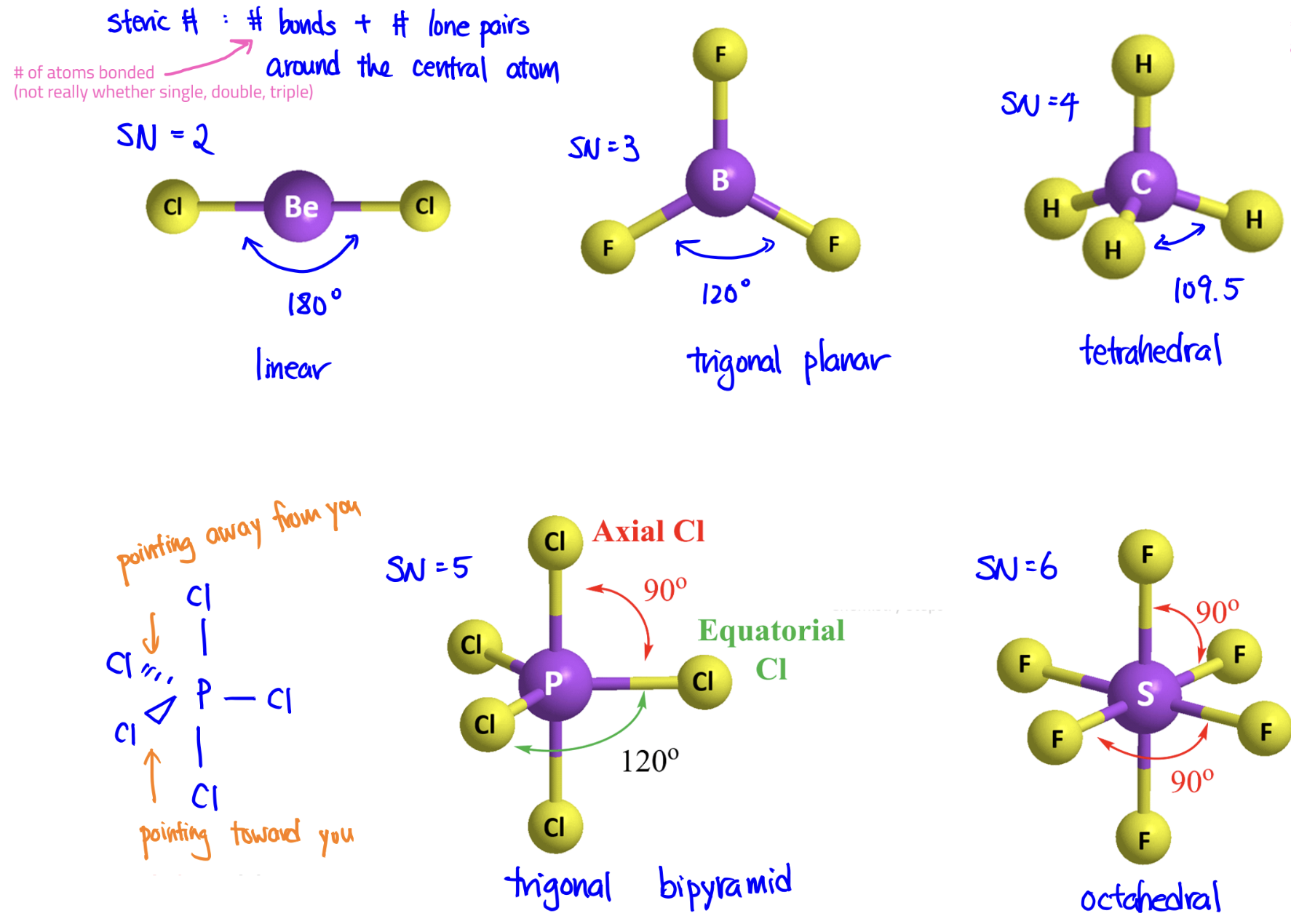
Due to repulsions, lone pairs and double bonds take up more space than single bonds and distort the geometry of the molecules.
Molecular Geometry Table
Polar covalent bond
a bond with a non-zero electric dipole moment
Polar Molecules
a molecule with a non-zero electric dipole moment
Nonpolar molecules
a molecule with zero electric dipole moment
A nonpolar molecule can have polar bonds
Steps for determining if a molecule is polar
Determine the shape of the molecule
Find all the polar bonds and draw the electric dipole moment
See if the polar bonds cancel each other out
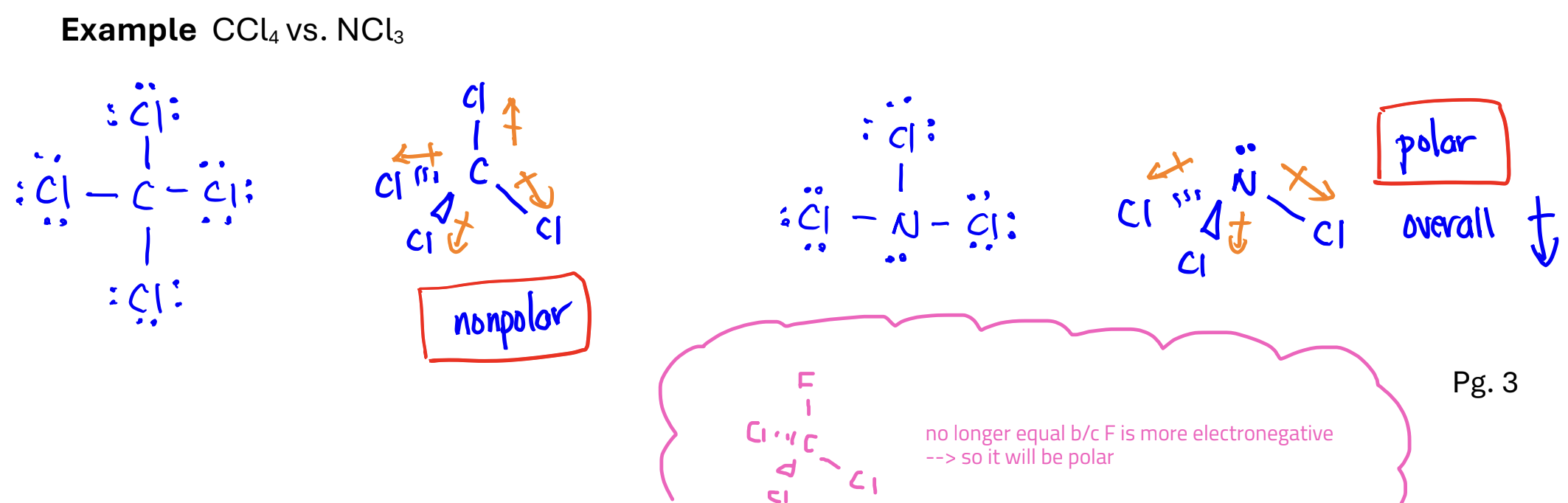
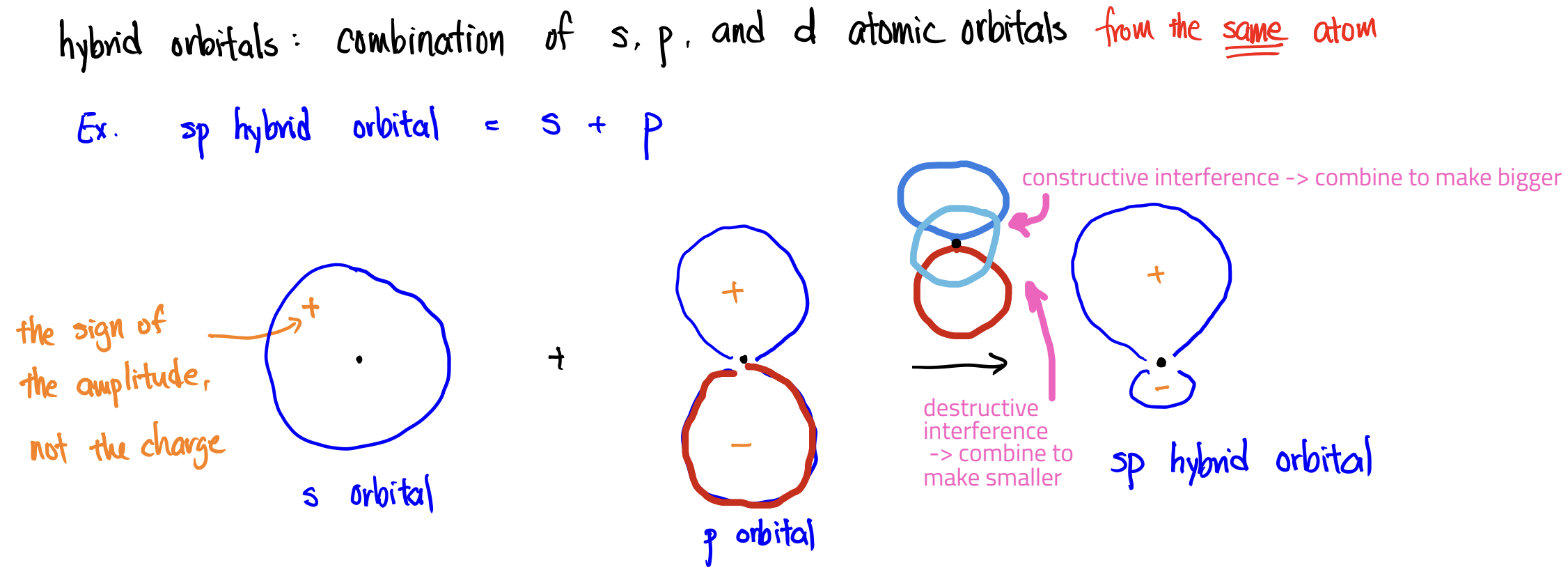
Valence Bond (VB) Theory
how we get bond angles & where are the e- in resonance structures
Hybrid Orbital
combination of s, p, and d orbitals from the same atom
SN = 2

SN = 3

SN = 4

SN = 5

SN = 6
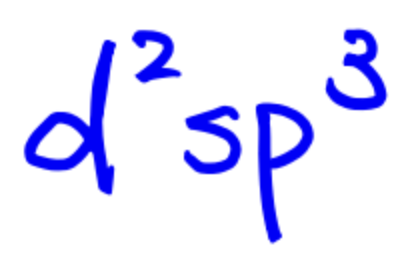
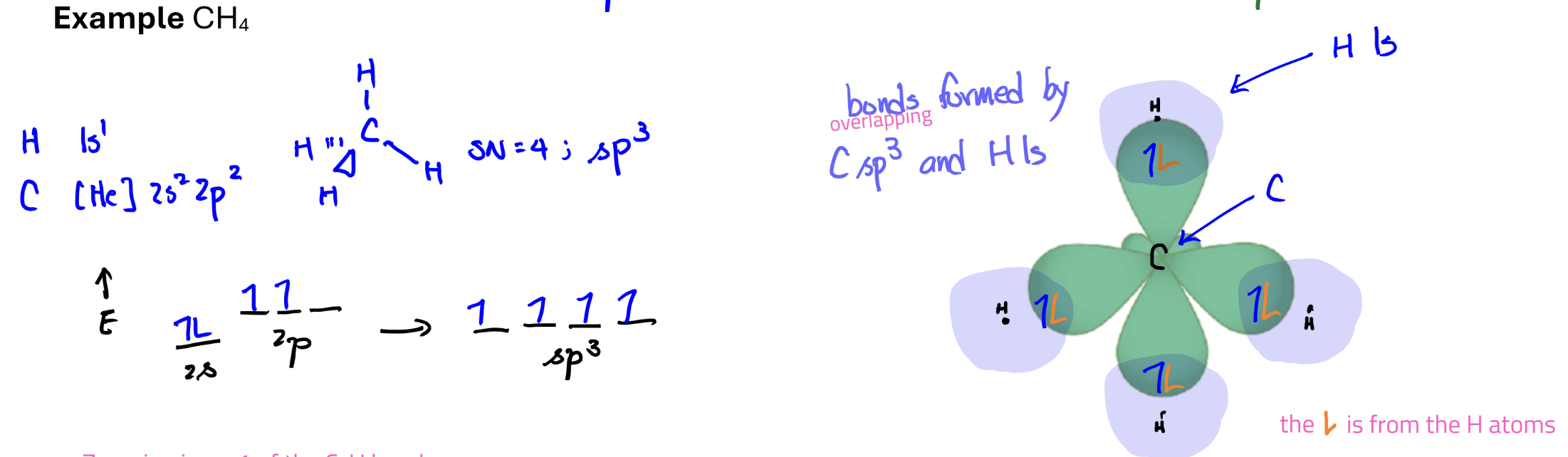
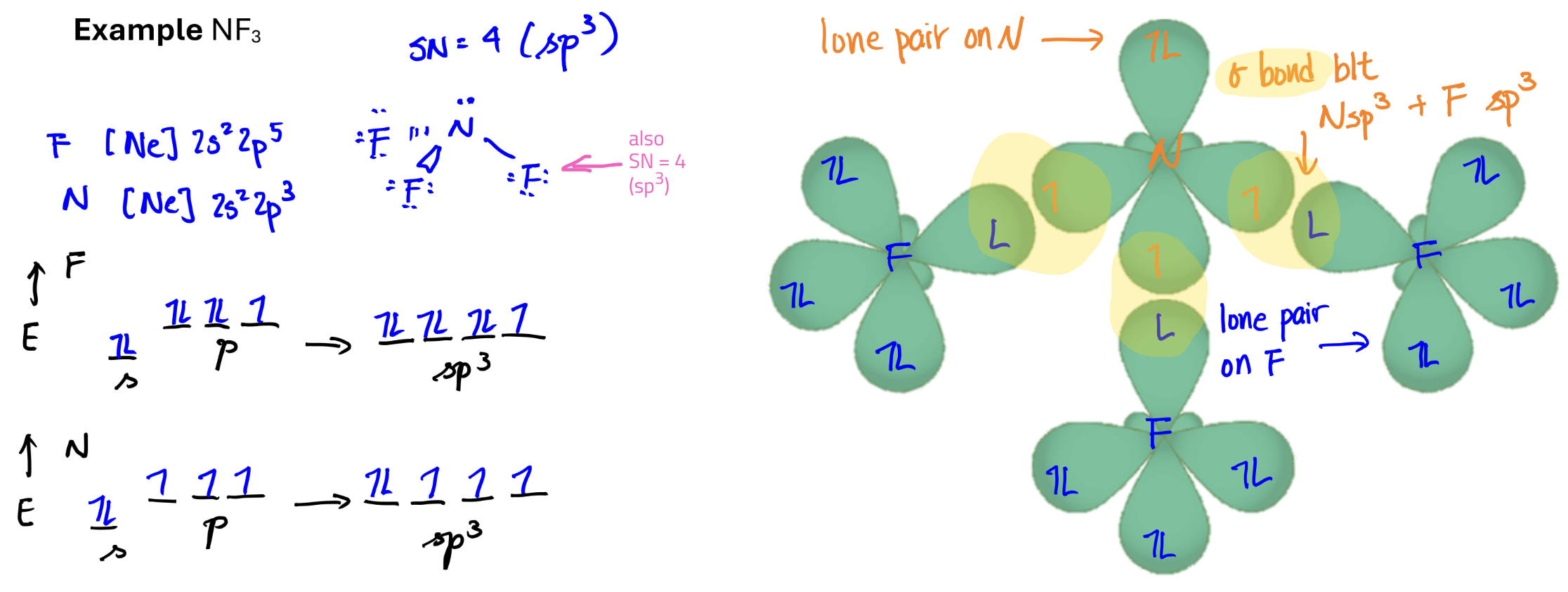
Electron Configuration and Bonding with Hybrid Orbitals
General rule:
Hydrogen atoms do not hybridize and use the 1s orbital to form bonds
All other atoms hybridize to form bonds
the # of orbitals used to combine = the # of hybrid orbitals
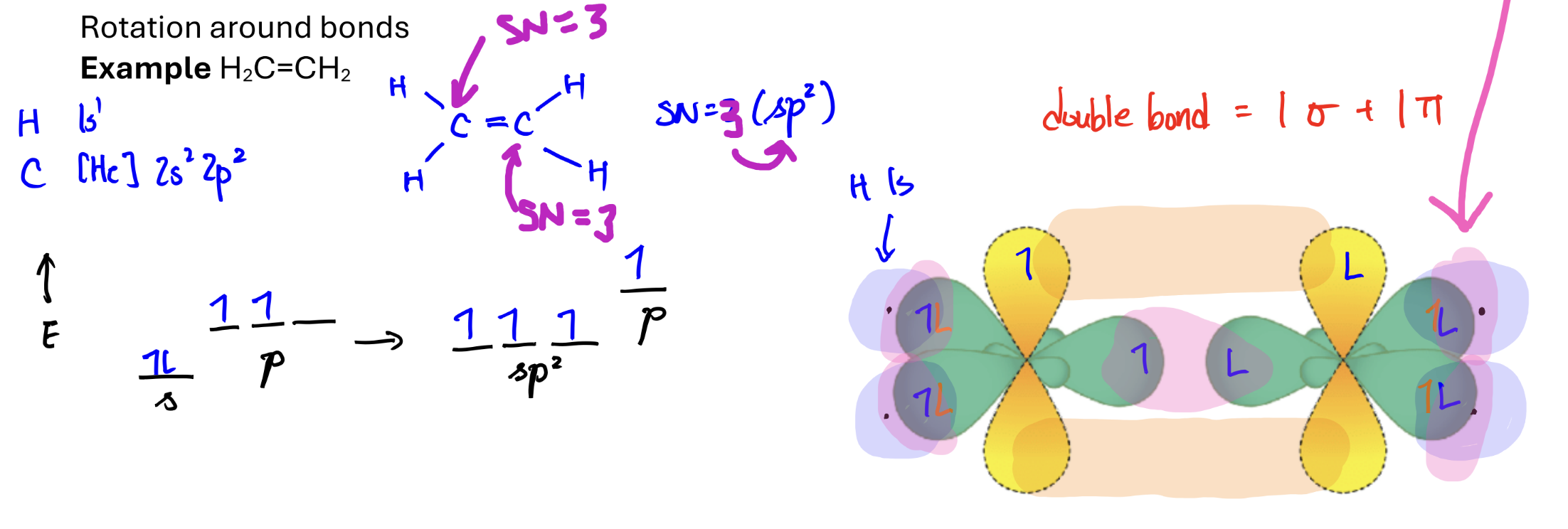
σ-bonds
formed by hybrid+hybrid or hybrid+ H 1s
Orbitals overlap end-to-end and electrons are b/w the nuclei
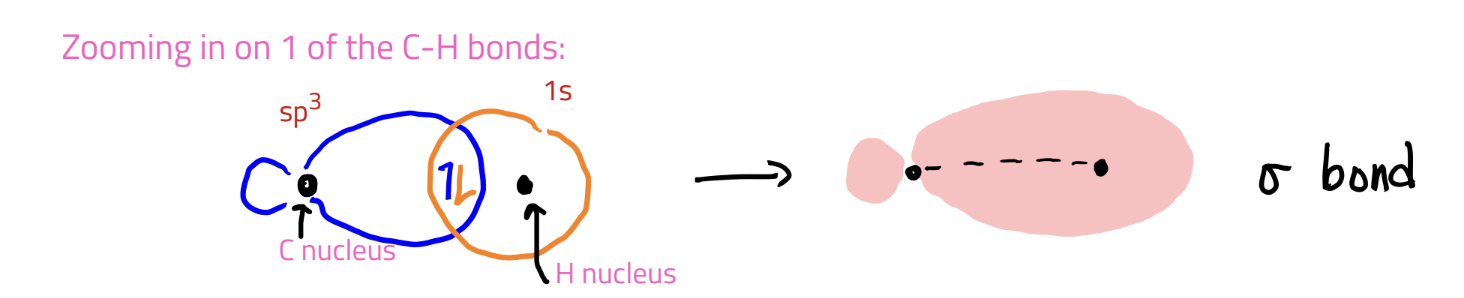
*non-bonding lone pairs are placed in the hybrid orbitals
π-bonds
formed by p orbital + p orbital
Orbitals overlap side-by-side and electrons are above/below the nuclei.

Note: conjugated π system = alternating single and double bonds
σ- and π- bonds in double and triple bonds
double bonds: 1 σ + 1 π
triple bonds: 1 σ + 2 π