IB Chemistry HL (2nd Edition) Chapter 6 : Chemical Kinetics
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Collision Theory
For a reaction to occur, the particles must collide, they must collide with the appropriate orientation, and they must collide with sufficient energy.
Rate
a measure, quantity, or frequency, typically one measured against some other quantity or measure.
Rate of reaction
The change in concentration of a reactant or a product in a given time.
Rate of reaction equation
amount of reactant used up or amount of product formed / time
kinetic energy
the energy an object has due to its motion
absolute temperature
Temperature measured on the Kelvin scale
activation energy (Ea)
the minimum amount of energy required to initiate a chemical reaction
Rate expression (rate law)
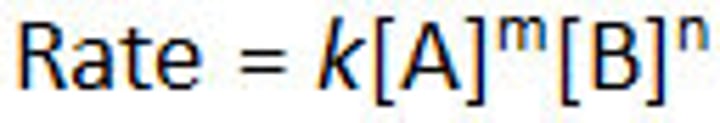
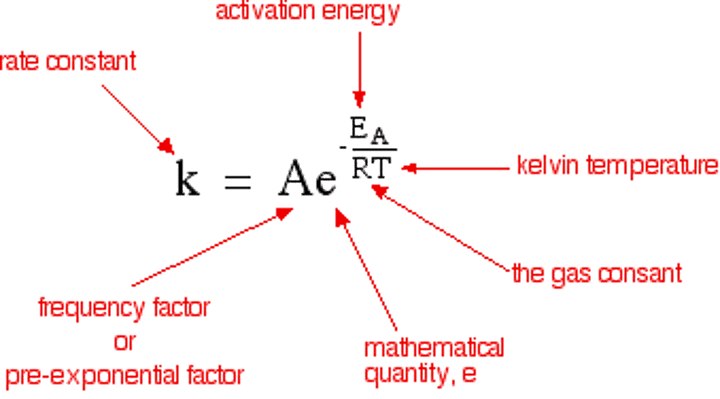
Arrhenius constant
Indicates the frequency of collisions and the probability that collisions have proper orientations
Arrhenius equation
Which of the following statements is correct?
A : A catalyst increases the rate of the forward reaction
B : A catalyst increases the rate of the forward and backward reaction
C : A catalyst increases the yield of product formed
D : A catalyst increases the activation energy of a reaction
B
A sugar cube cannot be ignited with a match, but a sugar cube coated in ashes will ignite. Suggest a reason for this observation.
The ashes must contain a catalyst that speeds
up the reaction between sugar and oxygen.
(Deduced from the fact that all other factors that
affect reaction rate can be ruled out.)
Catalytic converters are now used in most cars to convert some components of exhaust gases into less environmentally damaging molecules. One of these reactions converts carbon monoxide and nitrogen monoxide into carbon dioxide and nitrogen. The catalyst usually consists of metals such as platinum or rhodium.
a) Write an equation for this reaction
b) Explain why it is important to reduce the concentrations of carbon monoxide and nitrogen monoxide released into the atmosphere.
c)Why do you think the converter sometimes consists of small ceramic beads coated with the catalyst?
d) Suggest why the converter usually does not work effectively until the car engine has warmed up.
e) Discuss whether the use of catalytic onverters in cars solves the problem of car pollution.
(a) 2CO(g) + 2NO(g) → 2CO2(g) + N2(g)
(b) CO is a toxic gas: it combines with
haemoglobin in the blood and prevents
it from carrying oxygen. NO is a primary
air pollutant: it is oxidized in the air to
form acidic oxides, leading to acid rain.
It also reacts with other pollutants in the
atmosphere, forming smog.
(c) The increased surface area of the catalyst
in contact with exhaust gases will increase
catalyst efficiency.
(d) Catalytic activity involves the catalyst
interacting with the gases, and the reaction
occurring on its surface. As temperature
increases, the increased kinetic energy of
the gases increases the frequency with
which they bind to the catalyst.
(e) Catalytic converters reduce pollution from
cars but do not remove it completely. As in
(d), they are not effective when the engine
first starts from cold, when an estimated 80%
of pollution occurs. Other pollutants in car
exhausts are not removed by the catalyst,
e.g. ozone, sulfur oxides and particulates.
Also the catalytic converter increases the
output of CO2, a serious pollutant because of
its greenhouse gas properties.