DENT 730A Lecture 2: Bioenergetics and Carbohydrate Metabolism
1/80
Earn XP
Description and Tags
Flashcards cover key concepts from Lecture 2: Bioenergetics, electron transport chain, and carbohydrate metabolism, including glycolysis, TCA, gluconeogenesis, glycogen metabolism, PPP, sugar digestion/absorption, and glycan-containing biomolecules.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
81 Terms
What is Bioenergetics?
The study of energy transformations in biological systems; it PREDICTS whether a process is spontaneous (favorable) or non-spontaneous (unfavorable) based on Gibbs free energy change (ΔG).
What are the three factors that affect bioenergetics?
Enthalpy (ΔH), Entropy (ΔS), and Free Energy (energy avaiable to do chemical work) (T); ΔG = ΔH − TΔS.
Define ΔG, ΔH, and ΔS T
ΔG: change in free energy (energy available to do work); ΔH: change in enthalpy (heat released or absorbed); ΔS: change in entropy (disorder). and heat
What does a negative ΔG signify?
A spontaneous (favorable) reaction.
Difference between ΔG and ΔGo
ΔGo is the standard free energy change (@1 mol/L 298K and 1 ATM) (a baseline when comparing reactions under standard a standard state)
ΔG is free energy
Standard free energy of hydrolysis of ATP?
-7.3 kcal/mol
Explain the chemiosmotic hypothesis.
Energy from electron transport pumps protons across the inner mitochondrial membrane from matrix into INTERMEMBRANCE space creating a gradient from NADH and FADH2 donate a pair of electron protons flow back through ATP synthase (F1F0) to synthesize ATP (oxidative phosphorylation) (series of redox reaction)
Where is the electron transport chain located and Kreb Cycle?
ETC located in the inner mitochondrial membrane
Kreb Cycle - matrix
Complex 1
NADH Dehydrogenase enzyme transfer of electrons from NADH to the electron transport chain. (NADH is from Kreb Cycle)
E is transferred to CoQ
Complex 2
Does not Span the membrane but is attached
Succinate Dehydrogenase Succinate to Fumarate
Electrons from FADH2 transfered to Coenzyme Q
Coenzyme Q
Accepts E and proton from both complez 1 and 2 then transfers to Complex 3
Complex 3 and 4
E are passed to Cytochome C to complex 4 then at 4 it O2 is reduced to water. O2 is the final E acceptor
How many ATP are produced per NADH and per FADH2 in the ETC?
Approximately 2.5 ATP per NADH (1 NADH = 10 Proton and C4 = 4 Proton = 2.5 thus 10/4) and 1.5 ATP per FADH2.
What ROS are produced in the ETC and what enzymes defend against them?
Reactive oxygen species such as superoxide and hydroxyl radical can damage cells; defenses include superoxide dismutase, catalase, and glutathione peroxidase.
What is the ETC inhibited by?
Electron flow and ATP synthesis are inhibited because oxidative phosphorylation is tightly coupled to the ETC.( Amytal Rotenone, antimycin A and CN CO H2S NaN3)
Where is the normally more H+
More H+ in the intermembrance space so pumping H+ in there makes a strong proton gradient
Uncoupling Proteins (UCP)
Inner membrane allows H+ but NO ATP is made this energy is called heat and is released through a process known as non-shivering thermogenesis.
Racemases
Enzyme that can inverts D- and L-Isomers the furthest chircal carbon from hydroxl group determines the isomer
Digestion of Carbs Final Products
Final Products: Glucose, galactose and fructose are broken down to monosac and absorbed in small intest.
Where does digestion begin
Begins in mouth
Difference between Starch and Glycogen and its branches
Starch (Plants) can be in amylose (straight chain) or Amylopectin (branched)
Animal (glycogen)
How is cellouse broken down
By Salivary amylase breaking a(1’4) bonds but humans dont have B(1’4) thus we lack the enzyme endoglucosidase to break down it
How and what transport glucose into the cell
GLUT 2 found in pactreatic B cell and live and kidney transporter moving outside the cell
GLUT 4 is in adipose tissue and skelemuscle but is insulin dependent bringing it in
Sodium dependent glucose linked transporter (SGLT) pumps against concen gradient pumps fructose glucose and galatacose into epitehlia cells of the intestine
Abnormal Degradition of disaacharides
only monosac are digested and abnormalilties of dissach break down occurs leading to water being drawn causes diarreah and bacterial fermanation
Lactase Deficency
Lactase intolerance leading to lactose being unbroken into galactose + glucose which leads to bacteria fermneting this and relasing H2 which leads to methane (farts)
Carbohydrate Metabolism Chart
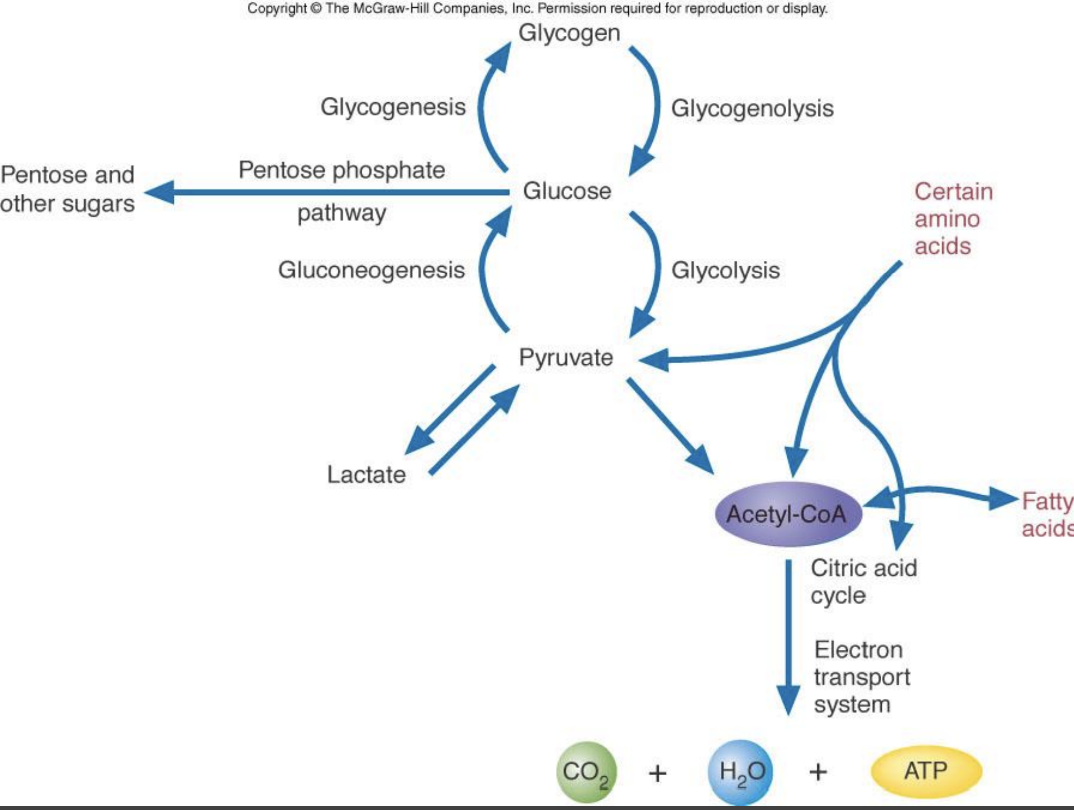
1st Irreversible step of Glycolysis
Hexokinase phosphorlyzes glucose turning it to G6P
Glucokinase is a derivative that has a higher vmax and km than hexokinase 1-3 allowing it handle more and lower affinity
these enzymes are inhibited by G-6-P thus preventing any feedback and committing it into cell membrane
Step 3 of glycolysis (reg. Step)
F6P into Fructose 1’6 by PFK-1 MOST important control point because it commits to glycolysis
What is the fate of pyruvate under aerobic conditions?
Pyruvate is converted to acetyl-CoA by the pyruvate dehydrogenase complex and enters the TCA cycle, generating NADH.
What are the components and cofactors of the Pyruvate Dehydrogenase (PDH) complex?
E1 (pyruvate dehydrogenase), E2 (dihydrolipoyl transacetylase), E3 (dihydrolipoyl dehydrogenase); cofactors include thiamine pyrophosphate (TPP), lipoic acid, CoA, FAD, and NAD+. Arsenic can inhibit lipoic acid–containing enzymes.
How does arsenic poisoning affect energy metabolism?
Affects step #6 (oxidation of G3P) where NADH is made. Asenic competes with Pi for G3P deydrogronease, which leads to 3-Phophoglveryate formation directly leading to NADH made but not ATP made losing an ATP pay off phase
Step 9 of Glycolysis
2-Phosphoglycerate is dhydrated to pep by Enolase
enolase can be inhibited by fluoride
Fate of Pyruvate
can go to aerobic metobolism and anaeorbic making lactate or ethanol
Step 10 (reg step)
Phosphoenolpyruate PEP is converted to pyruvate by the enzyme pryruatcute kinase which is irresible and ATP is made in this step too
Pyruvate Dehydrogenase
the enzyme that coverts pyruvate to acetly CoA when it enters the mitochondrial matrix NaDH is reduced and Co2 is released
PDH coenzymes deficiencies
E1 Pyruvate dehydrogenase can be deficient due to lack of TPP from B1 vitamins leading to pyruvate being shunted to lactic acid ferm which auses congential lactic acidosis
What is the rate-limiting step of the TCA cycle?
Isocitrate dehydrogenase is one of the rate-limiting steps that is irrevisble and catalyzes the conversion of isocitrate to alpha-ketoglutarate producing NADH and CO2
Oxidation of Succinate
Succinate is oxidized to fumarate by succinate dehydrogease and FAD is reduced to FADH2
ONLY Enzyme embedded in the inner mitochondrial membrane (attached to complex 2 of ETC)
What is the first committed step of the TCA cycle?
Citrate synthase catalyzes the condensation of acetyl-CoA and oxaloacetate to form citrate.
Highest regulated enzymes in TCA
Citrate synthease
Isocitrate dehydrogenase
a-ketoglutarate dehydrogenase complex
Where does gluconeogenesis predominantly occur during an overnight fast, and what percentage is in the liver?
Predominantly in the liver; about 90% in the liver and 10% in the kidneys.
What are the primary substrates for gluconeogenesis?
Glycerol, lactate, and glucogenic amino acids.
Which enzymes bypass irreversible glycolytic steps in gluconeogenesis?
Pyruvate carboxylase (REQUIRES ATP) to ooxaloacate then PEP carboxykinase bypass the pyruvate kinase step fructose-1,6-bisphosphatase bypasses PFK-1; glucose-6-phosphatase bypasses hexokinase/glucokinase.
What are the substrates for gluconeogenesis listed in the notes?
Glycerol, lactate, and amino acids.
Glycerol Kinase
Glycerol is converted to G3P and then to DHAP (both in glycolysis and glucneo) wihch is only in the liver and is not in Adipocytses because it lacks the enyzme.
Cori Cycle
Lactate is released into the blood by muscles and liver and through rbc which is converted by lactate dehydrogenase into glucose
amino acid as a substrate for gluconeogensis
Muscle protein like Alanine Serine Glycine Cysteine and Throeonine can be converted to NH3 then to glumatate to alanine which can enter gluconeo
amino acids are converted to oxaloacetate
Formation of glucose from G-6-P
Glucose 6 Phosphatase oonly found in live and kindney converts G6P to glucose
Reg. of Gluconeogensis
Horomoes such as glucagon from a-cells of pancreatic stimulate
Main stores of glycogen?
liver and skeletal muscle
fluctuation of glycogen
liver glycoen stores increase during the well fed state and are depleted during a fast
muscle glyocgen is not affected by short period of fasting and is synthesized to replensih muscle stores after a strenous excersise
What is glycogenesis and where does it occur?
syntheszizd from alpha d glucose involves G6P to G1P, UDP-glucose, glycogenin primer, glycogen synthase, and branching enzyme.and occurs in the cytosol
Phosphoglucomutase
synthesis of glucose 1-phosphate from G-6-P in glycogensis
Synthesis of UDP-glucose
UDP-glucose pyrophorylase converts glucose1-phosphate to UDP-glucose, which is more reactive than glucose
Glycogen Synthase
converts UDP-glucose to UDP which needs glycogenin as a primer and releases a glycogen
What enzyme forms branches in glycogensis
amylo a 1’4 and a(1,6)transglcosidase forms branches. 1’6 banches and removes a chain from non reduncing end after 6-8 residues added on by amylo
How is glycogen metabolism hormonally regulated in the liver and muscle?
Glucagon and epinephrine stimulate glycogenolysis; insulin stimulates glycogenesis. Glycogen synthase is inactivated by phosphorylation (kinases) and reactivated by protein phosphatase-1. Allosteric regulation aligns with energy/nutrient status.
Which enzyme cleaves off a(1’4) glycosidic chain
glycogen phosphorylase releases glucose at nonreudcing ends. You get glucose 1-phosphate + phosphate from it
What enzyme cleaves off 1 branch of 1’6
Amylo-a(1’6) glucosidase removes the single remaining releasing it as free glucose
How is g-6p converted in liver and muscles
g-6-p is conoverted to glucose my glucose-6-phosphatase in the liver and in the muslces g-6p cannot be dephosphoryed bc of the lack of the enzyme thus it ends glycolysis
Von gierke disease
a defienciy of glucose 6 phosphateases which cuases glycogen storage disease in type 1 diebetes leading to inability of liver to provide free glucose during a fast.
Regulation of Glycogen synthesis and degradation
Liver: Glycogenesis accelerates during periods where the body has been well fed
Glycogenolysis accelartes during periods of fasting
Muscle: glycogenolsis occurs during active exercise
glycogensis begins as soon as the muscle is again at rest
Hormonal regulation of Glycogen synthesis and degradation
glucagon stimulates glycogenlysis and inhibits glycogensis
epinephrine stimulates glycogenlysis and inhibits glycogensis
insulin stimulates glychoensis and inhibits glycogenolysis
Active Form of Glycogen synthesis and degradation
Active form of Glyocgen degradtion is when Glycogen Phosphorylase is activated by ATP
Deactive form is Glycogen phosphorlase B when Protein phosphate 1 removes the phosphate from a signal from insulin leading
how does glucose and galatose get into the cell
sodium -glucose transporter and leave by glut 2
how does fructose get into the cell
Glut 5
composition of Malatose, sucrose, lactose
malatose - two gluclose
sucrose - glucose + fructose
lactose - glucose + galatcose
what is Hexose monophosphate shunt
PP pathway
Glucose-6-Phosphate dehydroganse
Enzyme that catalyzes the conversion of glucose-6-phosphate to 6-phosphogluconate in PP pathway relasing NADPH
6-phosphateglucoante Dehydrogenase
Enzyme that catalzyed 6-phosphogluconate to ribulose 5 phosphate releasing NADPH in PPP
How is nucleic acid biosynthesis made
ribose 5 phosphate is the precurors when added with a 2c chain in PPP
NADPH function
used in fatty acid synthesis, reduction of h2O2, and reduction of one Oxygen to h2o, and maitain hemoglobin in its reduce form and protects agreats hemolysis
What is the most common disease causing enzyme abnormaily in humans
Glucose-6-phosphate dehydrogenase defiency which impairs the ability of RBC to form NADPH and this is done by glutatione reductase then go through Glutathione peroxidases to convert the oxidant stress of H2O2 and convert to 2H2o
h2o2 is detrimental to red blood cells, leading to hemolytic anemia when deficiency occurs.
What are glyococojungates
important molcules of the ECM that need to be turnover by lysosomes if not it could to defeictive degreading resultsing in storage diseases such as mucopolysacchirdoses and ologiosacchinesdose
What is the difference between GAGs, proteoglycans, and glycoproteins?
GAGs are linear, negatively charged polysaccharides in the ECM;
Proteoglycans are core proteins with GAG side chains;
Glycoproteins are proteins + oligosaccharide chains that are branched
What diseases are associated with degraded lysosomal hydrolases acting on GAGs?
Mucopolysaccharidoses (MPS) and oligosaccharidoses; accumulation of GAGs leads to skeletal/ECM abnormalities and other symptoms.
What is the Pentose Phosphate Pathway and what are its key products?
A pathway of glucose-6-phosphate in the cytosol that generates NADPH and ribose-5-phosphate; important for reductive biosynthesis and nucleotide synthesis.
Which enzymes are key in the oxidative phase of the PPP and what is the consequence of their deficiency?
Glucose-6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate dehydrogenase generate NADPH; G6PD deficiency impairs RBC protection from oxidative stress.
How are NADPH and ribose-5-phosphate used in cells?
NADPH provides reducing power for biosynthesis and antioxidant defense; ribose-5-phosphate is used for nucleotide synthesis.
What are the three classes of carbohydrate-derivative structures discussed and how do they differ?
Glycosaminoglycans (GAGs) – linear polysaccharides; Proteoglycans – core protein with GAG chains; Glycoproteins – proteins with short oligosaccharide chains.
What is the role of anomeric carbon and the difference between α- and β-anomers in cyclized monosaccharides?
Cyclization creates a new chiral center at the anomeric carbon (C1 for aldoses). α- and β- refer to the position of the anomeric hydroxyl relative to the CH2OH group (α typically on the opposite side to CH2OH in Haworth projection; β on the same side).