C2.7 Metallic bonding
0.0(0)
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
4 Terms
1
New cards
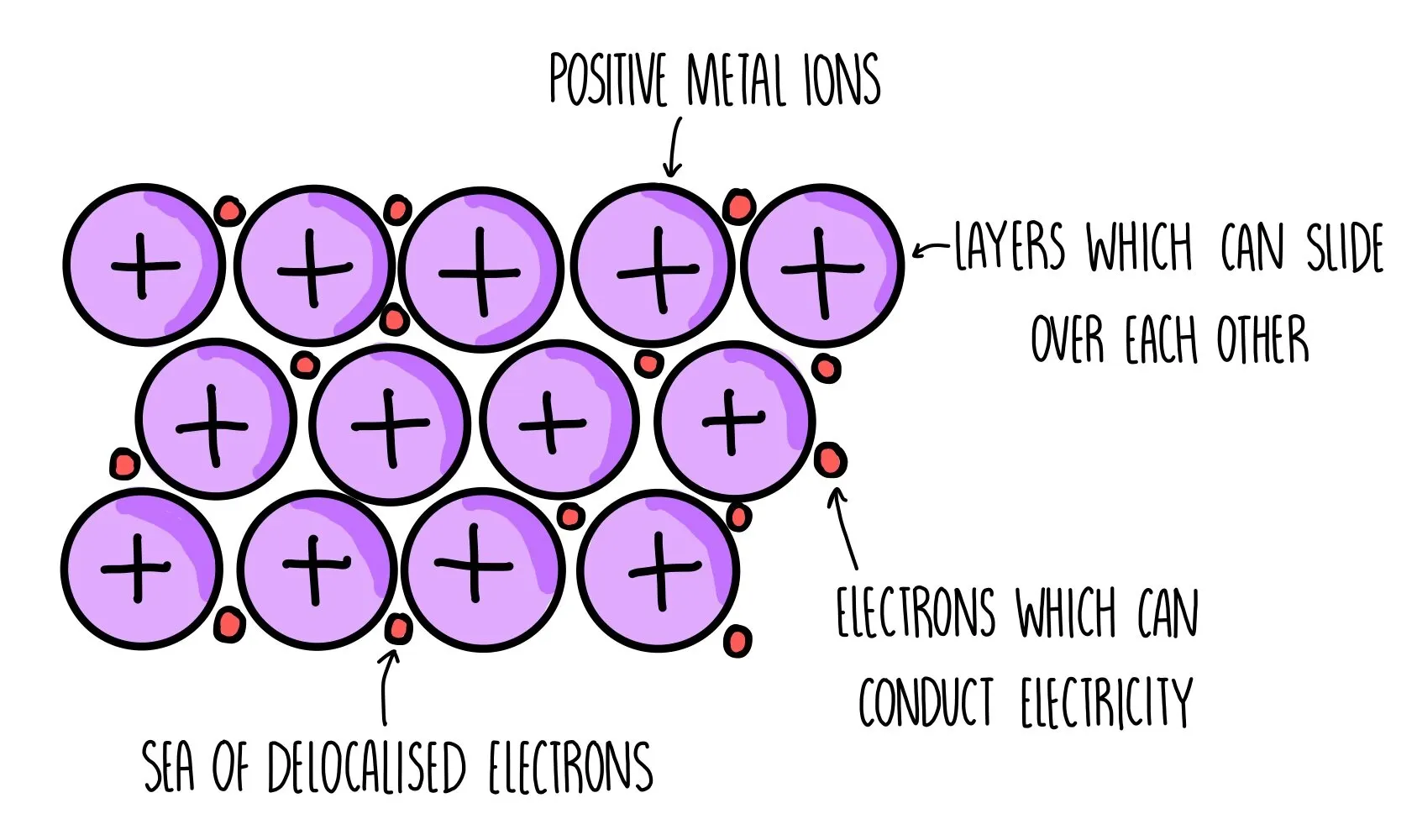
What is metallic bonding?
The electrostatic attraction between the positive ions in a giant metal lattice and a sea of delocalised electrons
Amount of delocalised electrons depends on the group number
2
New cards
Properties of metallic bonding
Good conductors of electricity: they have delocalised electrons which allow them to carry electrical charge
Malleable: the cations are arranged in layers that can slide over each other
High melting and boiling point: they have strong electrostatic attraction between cations and delocalised electrons which require a lot of energy to break
3
New cards
.
4
New cards
.