Quantum Physics - CAIE
1/6
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
Define the photon
A quantum of energy of electromagnetic radiation
What is the photoelectric Effect [2 marks]
Emission of electron
When EM radiation is incident (on surface)
Define the work function [2 marks]
The minimum photon energy required to remove an electron from the surface of a metal.
Define the De Broglie Wavelength
The wavelength associated with a moving particle
Explain why the maximum kinetic energy of the electron emitted from a metal surface is independent of the intensity of the incident electromagnetic radiation. [3 marks]
The frequency of a photon determines its energy.
The intensity of light determines the number of photons per unit time.
The intensity of light does not determine the energy of a photon.
The kinetic energy of an electron depends on the energy of one photon.
What is the equation linking momentum of a photon and its energy?
p = E/c, where c = speed of light in a vacuum.
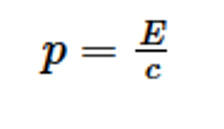
Explain how the emission spectrum provides evidence for the existence of discrete energy levels for electrons in an atom [3 marks].
Energy of photon has a corresponding frequency
change in electron energy level emits a single photon
photon energy = difference in energy levels
discrete frequencies must have come from discrete energy gaps.
discrete energy changes imply discrete energy levels.