Cell Death
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
65 Terms
What can force cells to die?
Severe insults such as ischemia (lack of blood flow) or exposure to poisons.
Why do cells die in a programmed way?
To eliminate cells that pose a danger or are no longer needed — for example, during immune defense or development.
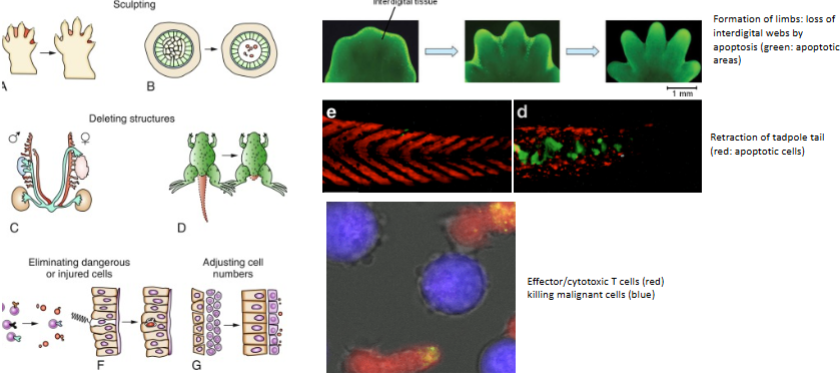
Which immune cells can trigger death of other cells?
Effector/cytotoxic T cells
What is necrosis
A biologically uncontrolled process - accidental cell death
How does regulated cell death differ from necrosis
Give example of regulated cell death
It is controlled by defined signalling cascades and effector mechanisms.
E.g. apoptosis
What necrotic-like cell deaths are regulated - name 3
Necroptosis, pyroptosis, and NETosis.
What normal physiological process involves apoptosis?
Keratinocyte terminal differentiation.
What are the 3 main types of regulated cell dath
Apoptosis
Pyroptosis
Netotic cell death
What happens which leads to apoptosis
DNA fragmentation
Loss of mitochondria function
What happens which leads to pyroptosis
Membrane rupture
What happens which leads to Netotic cell death
Histone citrullination
List the key morphological changes of apoptosis that occur in a sequential manner
• Blebbing
• Cell shrinkage
• Nuclear fragmentation
• Formation of apoptotic bodies
• Phagocytosis of apoptotic bodies
List key biochemical changes in apoptosis.
• Caspase activation (execution of the cell death programme)
• Degradation of cytoskeletal proteins (blebbing, nuclear fragmentation)
• Exposure of phosphatidyl serine on the cell surface (inducing phagocytosis)
• DNA fragmentation (unrepairable DNA damage)
• Loss of mitochondrial membrane potential (and ATP production)
What enzymes execute apoptosis?
Caspases — cysteine-dependent aspartate-specific proteases
What are the two main types of caspases?
Initiator caspases& executioner caspases
What are the main functional domains of caspases?
Pro-domains (for protein–protein interactions) and catalytic subunits (for enzymatic activity).
Name a protein-protein interaction that would happen on a caspase structure
Apaf-1 – pro-caspase-9 interaction
Name some targets of caspase cleavage (execution of apoptosis by cleaving essential proteins)
Caspase-dependent DNase
Lamin
Bcl-2
Mitochondrial complexes
What is the Bcl-2 protein family?
B cell lymphoma 2 protein
A family of proteins that regulate mitochondrial apoptosis
Name the three main groups of Bcl-2 proteins
Anti-apoptotic: Bcl-2, Bcl-XL, Mcl-1 (contain 4 BH domains: BH1–4).
Multidomain pro-apoptotic: Bax, Bak (contain 3-4 BH domains: BH1–3).
BH3-only pro-apoptotic: Bad, Bik, Bim, Bid, Puma, Noxa, Hrk
What stresses can trigger Bcl-2–mediated apoptosis
ER stress, DNA damage, and growth factor deprivation.
What are death receptors?
Single transmembrane domain receptors without enzymatic activity that mediate apoptosis upon ligand binding.
What ligands bind to death receptors?
Members of the TNF ligand family — Fas ligand, TNF, and TRAIL (TNF-related apoptosis-inducing ligand)
What domain do death receptors use to signal apoptosis
The death domain, which recruits adaptor proteins to propagate apoptotic signalling.
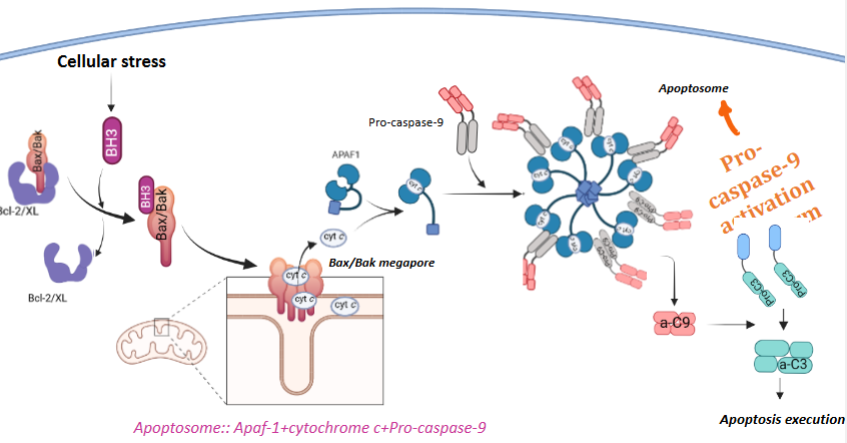
What triggers the intrinsic apoptosis pathway?
Signals originating from inside the cell that indicate severe cellular stress and unrepairable cellular damage initiate the intrinsic pathways of apoptosis.
Unrepairable cellular damage e.g. endoplasmic reticulum stress - accumulation of unfolded or misfolded proteins in the ER
What are the steps of the intrinsic apoptosis pathway
Cellular stress leads to activation of BH3 pro-apoptotic proteins
BH3 inhibits Bcl-2/Bcl-XL & activates Bax & Bak
Bax and Bak form megapores in the mitochondrial outer membrane - Mitochondrial outer membrane permeabilization (MOMP)
MOMP allows cytochrome c to released from the mitochondria into the cytosol.
Cytochrome c + APAF 1 + Pro-caspase-9 assemble to form the apoptosome
Pro-caspase-9 is activated in the apoptosome
Activated caspase-9 (a-C9) activates executioner caspases (e.g. a-C3) → apoptosis execution
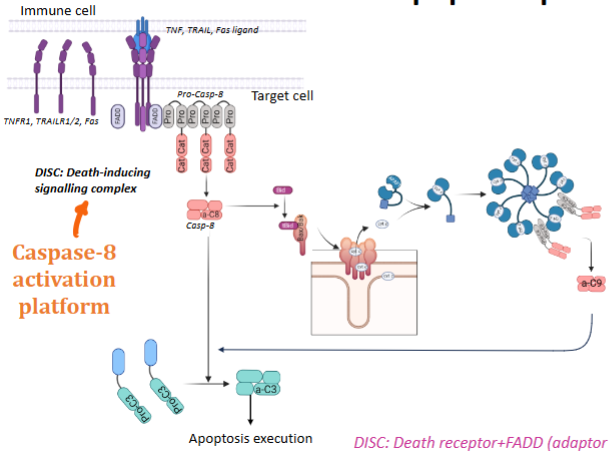
Explain the steps of the extrinsic apoptosis pathway
Immune cell presents a death ligand (Fas, Tumor necrosis factor (TNF), TNF-related apoptosis-inducing ligand (TRAIL)) to the receptor (part of tumour necrosis factor receptor family)
The receptor recruits adaptor proteins (FADD) and procaspase-8 to form the DISC (Death-Inducing Signalling Complex)
Within the DISC, procaspase-8 is activated → caspase-8 (caspase-8 activation platform)
Caspase 8 activates caspase 3
Steps 3 & 4 with the help if cytochrome c leads to the caspase enzyme cascade (this is the amplification system)
Caspase substrate proteins are the substrate of apoptosis
Biological response = cell death (or can also be inflammation / cell differentiation)
Cell lysis process
Release of perforin
Plasma membrane permeabilisation
Loss of osmotic homeostasis
Necrosis/cell lysis
TNF receptor 1 belongs to a superfamily of receptors called
Death Receptors
Death domain superfamily includes proteins with what 4 domains

What kind of interactions do domains in the death domain superfamily form
Domains form homotypic interactions, and occasionally heterotypic interactions
Does the death domain superfamily include both receptors & adaptors? Is that all it includes
Receptors
Adaptors
Enzymes
Name a receptor that has a death domain
TNFR1
TNFR1 signalling is mediated by recruitment of which adaptor protein
TRADD
Does TNFR1 have intrinsic enzymatic activity?
No — TNFR1’s intracellular domain lacks intrinsic enzymatic activity.
It contains the DD (death domain) instead
How is TNFR1 signalling initiated?
Homotypic interactions between the DD (death domain) of TNFR1 and DD- containing adaptor protein TRADD activates signalling
What event occurs when TNFR1 binds its ligand (TNF)?
Ligand binding induces trimerisation of the TNFR1 receptor - 3 signalling pathways are activated
What are the 3 major signalling pathways activated by TNFR1?
NF-κB (Nuclear factor–kappaB) and survival pathway
MAPK (JNK) pathway
Cell death (apoptotic) pathwa
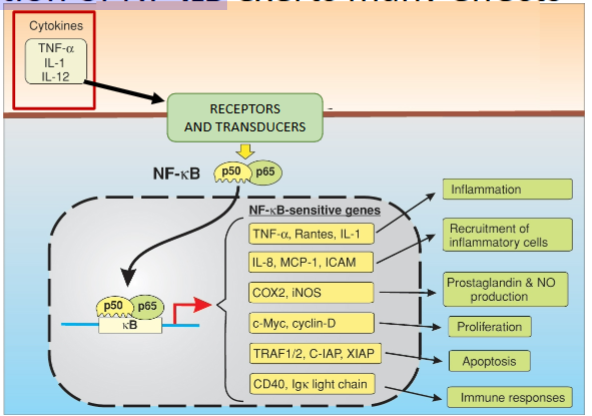
What is NF-κB?
A family of homo & heterodimeric transcription factors that regulate genes involved in inflammation, immunity, and cell survival.
What is the most common NF-κB dimer in canonical signalling?
The p50/p65 dimer
How does TNFR1 activation lead to NF-κB activation?
DD of TNFR1 recruits other DD proteins (e.g. TRADD) through homotypic interactions
TRADD acts as a platform for the recruitment of a signalling complex called Complex I that leads to activation of NF-kB
What does TRADD recruit by the TRADD recruitment platform to Complex I
TRADD recruits:
1. RIP1 through homotypic DD interactions (RIP=RIP1=RIPK1)
2. TRAF2 which recruits c-IAP
What is the function of cIAPs in Complex I?
They are E3 ubiquitin ligases that ubiquitinate target proteins to promote downstream signalling.
What does the BIR1 domain of cIAP do?
Mediates interaction with TRAF2.
What does the RING domain of cIAP do?
Provides E3 ligase activity.
What are the 3 main types of ubiquitination that happen to substance K in TNFR1 signalling?
K48-linked ubiquitination → proteasomal degradation.
K63-linked ubiquitination → signalling
Linear (head-to-tail) ubiquitination → signalling proteosomal degradation
What two kinase complexes are recruited to ubiquitin scaffolds in Complex I?
cIAP ubiquitinates itself and RIP1
Role of K63 polyUb chain in complex I
The K63 polyUb chains act as platforms for recruitment of downstream signalling molecules
In recruiting of kinase complexes, what is the role of the RIP1 polyUb chain & the c-IAP polyUb chain
RIP1 polyUb chain acts as a scaffold for TAK1/TAB kinase complex
c-IAP polyUb chain acts as a scaffold for IKK complex (I-kB kinase (IKK) complex comprising NEMO, IKK1, IKK2) & LUBAC (Linear Ub chain assembly complex)
Process of Phosphorylation of I-κB
TAK1/TAB kinase complex activates the IKK complex by phosphorylating IKK1
IKK phosphorylates I-κB
How is NF-κB kept inactive in the cytosol
Binding to IkB
What happens when IκB is phosphorylated?
It is tagged with K48-linked polyubiquitin and degraded by the proteasome.
What happens after IκB degradation?
NF-κB translocates into the nucleus, where it activates transcription of pro-survival genes.
Name 6 effects of activation of NF-kB
How does TNF-R1 signalling lead to cell death
TRADD recruits FADD
FADD recruits pro-caspase-8
Proximity-induced auto- activation of caspase-8
Activation of downstream caspases
Cell death
What two key factors determine whether TNF-R1 signalling leads to cell survival or death?
Timing of recruitment of specific components to the complex
Location in the plasma membrane
How does timing influence TNF-R1 signalling outcome?
Early recruitment of pro-survival complexes (Complex I) favours NF-κB activation and cell survival
Delayed or altered recruitment favours formation of pro-death complexes (Complex II) leading to apoptosis.
What in complex I inhibits caspase-8 binding to complex 2
cFLIP
What in complex I inhibits activity of caspases including caspase-3
XIAP
How does receptor location in the plasma membrane affect TNF-R1 signalling?
TNFR clustering within lipid rafts promotes NF-κB activation and survival
Localisation in non-raft regions favours apoptosis and death signalling.
How is TNF signalling implicated in autoimmune diseases like rheumatoid arthritis?
Excess TNF causes chronic inflammation, leading to diseases such as rheumatoid arthritis.
What are 3 examples of anti-TNF therapeutics and their mechanisms
Infliximab: Chimeric human-murine monoclonal antibody that binds and inhibits TNF-α.
Adalimumab: A recombinant human monoclonal antibody that binds and inhibits TNF.
Etanercept (Enbrel): Soluble TNFR-Fc fusion protein that inhibits TNF-α from binding to cell surface TNF receptors.
What are the steps involved in Regulated necrosis: NETosis
1. Pathogen phagocytosis
2. Oxidative stress/reactive oxygen species production
3. DNA de-condensation (dissociation of histones)
4. Gasdermin activation
5. Membrane permeabilization
6. Release of DNA net
7. Capture of extracellular pathogen by DNA
8. pathogen-DNA globule clearance by professional phagocytes (e.g. macrophages
Why might inhibition of pyroptosis components, such as PRRs and gasdermins be used clinically
Pyroptosis may result extensive cytokine and DAMP (damage-associated molecular pattern molecule) release
This results in immune overactivation
Overactivated immune cells attack non-damaged/non-infected cells causing tissue damage
E.g.: sepsis, Autoimmune and inflammatory diseases: Crohn’s disease, automimmune arthritis, colitis, multiple schlerosis
Inhibition of pyroptosis components, such as PRRs and gasdermins can reduce tissue damage and are in current testing for the therapy of chronic inflammatory conditions