Edexcel Chemistry: Core Practicals
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
97 Terms
How do you measure mass accurately?
1) Measure mass on 2 or 3d.p. balance of a weighing boat with the required quantity of solid in it
2) Empty mass into reaction vessel/flask
3) Reweigh the now empty weighing bottle
4) Subtract the mass of the empty weighing bottle from the first reading to give exact of mass actually added
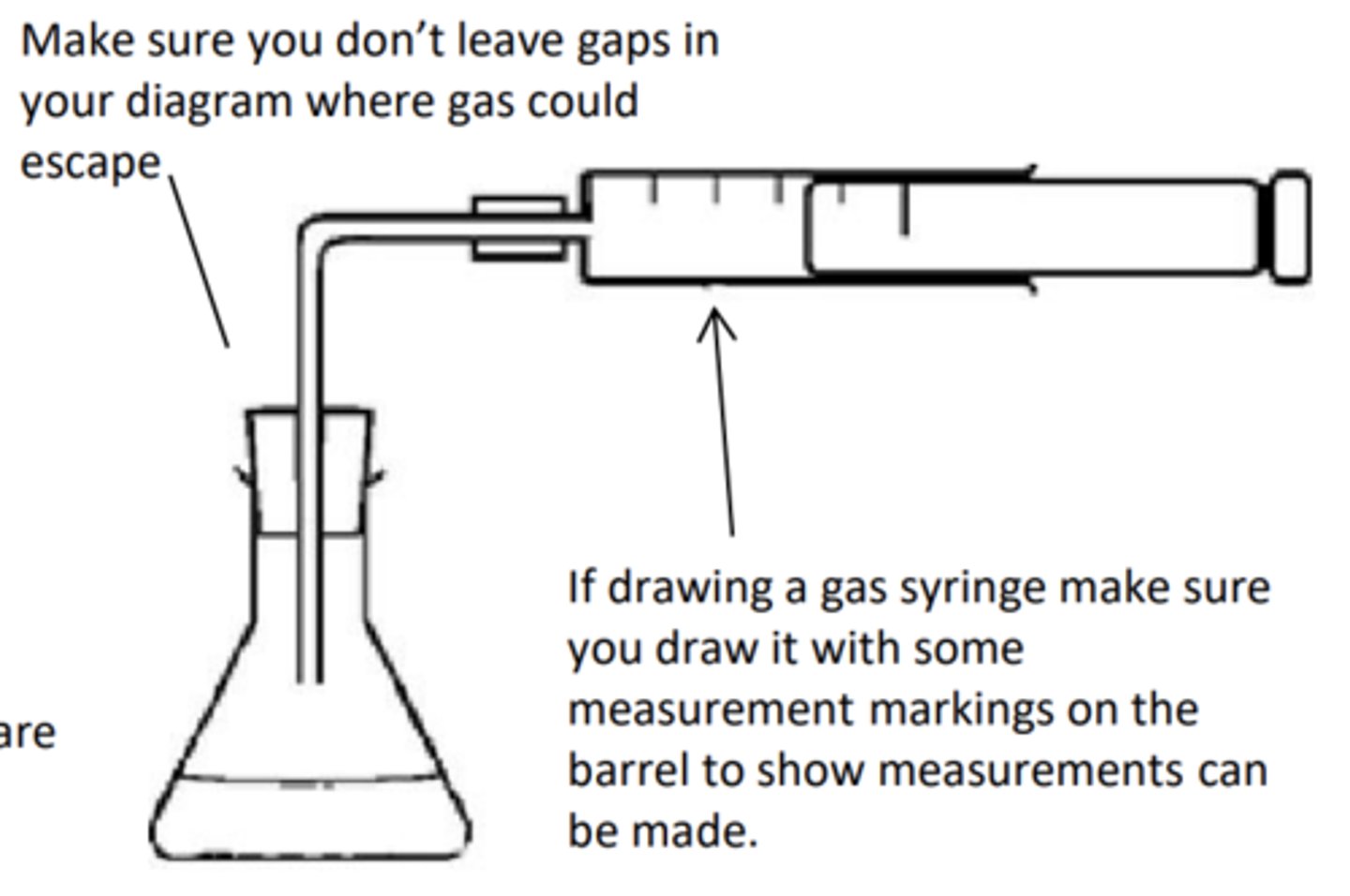
How would you carry out an experiment to measure the molar volume of a gas and draw it?
1. Place 30 cm3 of 1.0 mol dm-3 ethanoic acid into a conical flask
2. Attach conical flask to gas syringe
3. (Weight accurately) Place approximately 0.05 g of calcium carbonate in a weighing boat
4. Make a note of the mass of the weighing boat and its contents.
5. Remove the bung from the conical flask and tip the calcium carbonate into the conical flask. Quickly replace the bung in the conical flask
6. Measure the volume of gas at regular intervals measure and the volume of gas collected in the gas syringe once the reaction is over
7. Repeat the experiment six more times, increasing the mass of calcium carbonate by about 0.05 g each time. Do not exceed 0.40 g of calcium carbonate.
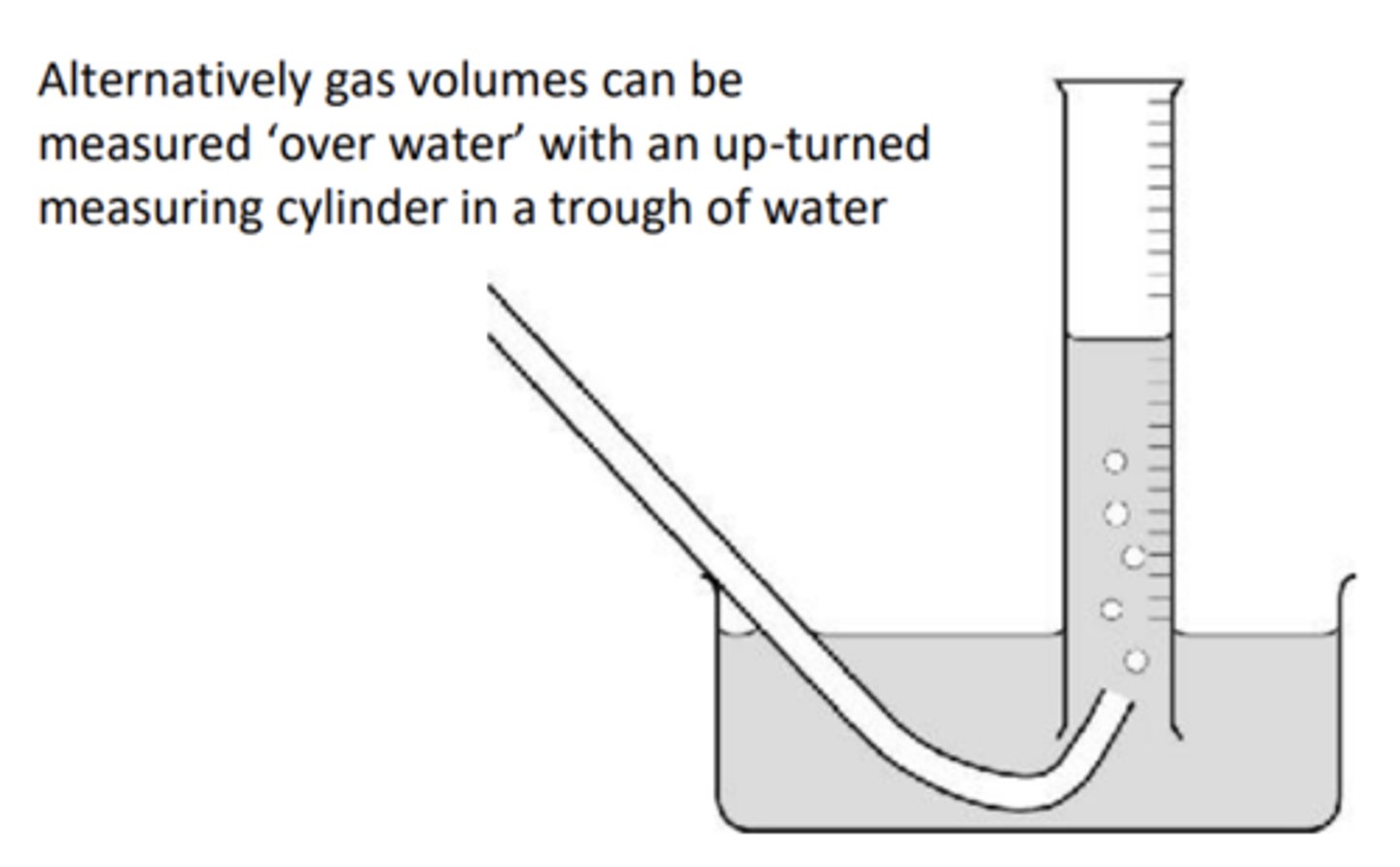
Draw the collection over water method and how do you use results to work out moles of gas
1) The volume of a gas depends on pressure and temperature so when recording volume it is important to note down the temperature and pressure of the room
2) PV = nRT or using the molar gas volume (1mol gas =24dm3 at room temperature and pressure)
Why is it more accurate to weigh the sample in the way described above?
As if it were tipped out some residue may be left in the sample tube so it would not be in the final mass which could reduce accuracy in the final result
What are the main sources of error in the previous experiment?
- Balance only measure to the nearest 0.01g
- Bung not replaced quickly enough so gas is lost during step 5
- Some gases like carbon dioxide or sulphur dioxide are soluble in water so the true amount of gas is not measured
From the given graph how would you work out molar volume of gas collected?
1) From the graph read the volume of CO2 given off with 0.25 g CaCO3
2) Work out the moles of CaCO3 in 0.25g = 0.25/100.1 = 2.5 x 10-3
3) Assume the moles of CO2 = moles of CaCO3
4) Work out molar volume of CO2 = volume of CO2 / moles of CO
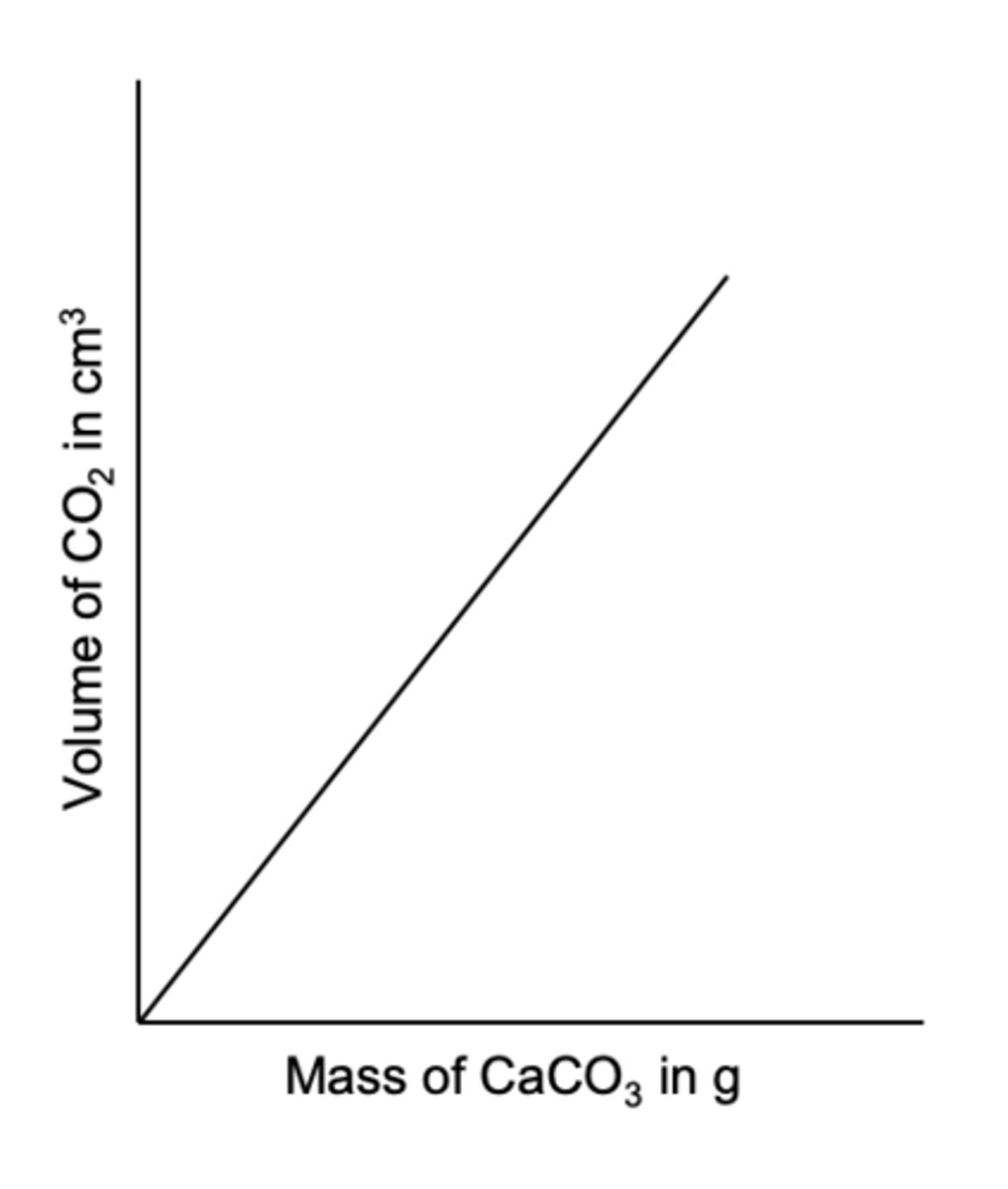
Why should you not exceed 0.40g of CaCO3?
Using more than 0.40g may cause the volume of gas that would be produced to exceed the maximum capacity of the gas syringe
How would you carry out an experiment to remove water vapour by heating as shown in the following equation?
CaSO4 .xH2O(s) → CaSO4 (s) + xH2O(g)
1) Weigh an empty clean dry crucible and lid
2) Add 2g of hydrated calcium sulphate to the crucible and weigh again
3) Heat strongly with a Bunsen for a couple of minutes 4) Allow to cool
5) Weigh the crucible and contents again
6) Heat crucible again and reweigh until you reach a constant mass (do this to ensure reaction is complete)
Why should you not use large amounts of CaSO4?
As the decomposition is likely to be incomplete
Why should you not use small amounts of CaSO4?
Percentage uncertainties in weighing will be too high
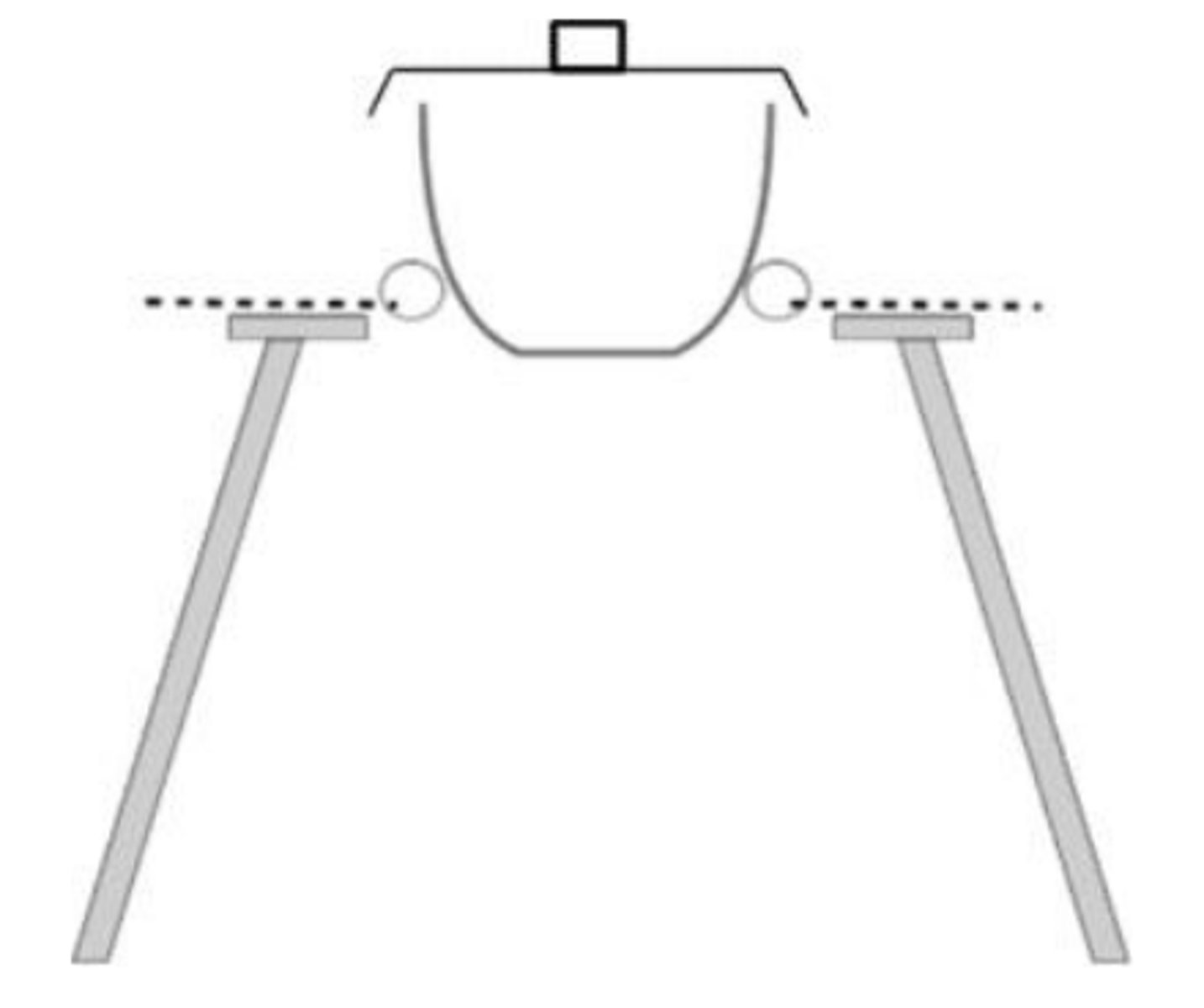
Draw heating with a crucible.
Why is a lid added?
Lid improves the accuracy of the experiment as it prevents loss of solid from the crucible but should be loose fitting to allow gas to escape
How do you prepare a standard solution?
1. Weigh out specified mass of compound into small beaker
2. Weigh the mass of this small beaker and contents accurately
3. Transfer contents into large beaker carefully. Weigh small beaker again
4. Add de-ionised water cautiously down the side of the large beaker, using about 150 cm3 of water. Swirl beaker to mix contents
5. Stir using a glass rod
6. Transfer the solution into the volumetric flask using a funnel, pouring down the glass rod and removing the last drop of solution from the glass rod
7. Wash the beaker, rod and funnel several times with deionised water, letting the washings go into the flask
8. Make up to the mark on the volumetric flask with deionised water so the meniscus sits on the line on the neck
9. Stopper firmly and shake the flask thoroughly
Why are mixtures shaken after they are made?
To ensure a uniform solution is made
How do you dilute a solution?
1) Pipette 25cm3 of original solution into a 250cm3 volumetric flask
2) Make up to the mark with distilled water using a dropping pipette for last few drops
3) Invert flask several times to ensure uniform solution
How should a titration be carried out?
1) Rinse equipment (burette with acid, pipette with alkali, conical flask with distilled water)
2) Pipette 25 cm3 of alkali/acid into conical flask
3) Touch surface of alkali/acid with pipette ( to ensure correct amount is added)
4) Add acid/alkali solution from burette
5) Add a few drops of indicator and refer to colour change at end point
6) Use a white tile underneath the flask to help observe the colour change
7) Add acid/alkali to alkali/acid whilst swirling the mixture and add acid drop wise at end point
8) Note burette reading before and after addition of acid
9) Repeat titration until at least 2 concordant results are obtained - two readings within 0.20cm3 of each other
When can distilled water be added to the conical flask and why?
- To wash the sides of the flask so that all the acid on the side is washed into the reaction mixture to react with the alkali
- Does not affect the titration reading as water does not react with the reagents or change the number of moles of acid added
Why should the funnel be removed from the burette during a titration?
As small drops of liquid may fall from the funnel during the titration leading to a false burette reading, a lower titre
Where does the substance of unknown concentration go and how are their volumes measured?
In the conical flask using a volumetric pipette
When should phenolphthalein and when should methyl orange be used as the indicator and what are their colour changes?
- Methyl orange: acidic neutralisation end-points when alkali is in the conical flask: red to yellow
- Phenolphthalein: basic neutralisation end-points when acid is in the conical flask: colourless to pink
What is a source of inaccuracy in titrations?
Make sure the jet space in the burette is filled with acid as it will lead to errors if it then fills during the titration, leading to a larger than expected titre reading
Why is it better to have a large titre?
The percentage error will be smaller, the larger the titre
How is % uncertainty calculated?
% uncertainty = uncertainty / measurement made on apparatus x 100
How can the % uncertainty be improved?
Decrease the sensitivity uncertainty by using apparatus with a greater resolution (finer scale divisions) or you can increase the size of the measurement made
How would you carry out an experiment to investigate the hydrolysis of halogenoalkanes?
1) In separate TT add 1cm3 of haloalkanes and 1cm3 of ethanol
2) Ethanol ensures that RX dissolves and can react with H2O nucleophile
3) Place in water bath
4) When RX have reached temperature of H2O in water bath add silver nitrate and start stopwatch
5) Time how long it takes for ppt to form
What is the reaction that occurs during the hydrolysis of haloalkanes, using iodoethane as an example?
What is the reaction that occurs to produce the ppt?
- CH3CH2 I + H2O → CH3CH2OH + I - + H+
- Ag+ (aq) + I - (aq) → AgI (s)
Why can H2O be used as a nucleophile?
Water has lone pair(s) of electrons on the oxygen atom
What haloalkane has the slowest rate of hydrolysis?
Chloroalkanes as as the C-Cl bond is weakest and so it hydrolyses the quickest. This is the case because C-Cl bond is strongest as it is the smallest halogen atom so the harder it is to break and the slower the reaction
How would you carry out an experiment to investigate the oxidation of ethanol?
1. Place about 10 cm3 of dilute sulphuric acid in a flask and add about 3g of potassium dichromate(VI) and 2 or 3 anti-bumping granules. Shake the contents of the flask until solution is complete.
2. Cool the flask in an ice-water bath
3. Set the flask up for reflux keeping it in the ice-water bath
4. Measure out 1 cm3 of ethanol
5. Using a pipette, add the ethanol a few drops at a time down the reflux condenser. This must be done slowly. Allow for the reaction to subside after each addition before adding more
6. When all of the ethanol has been added, remove the ice-water bath and allow to warm to room temperature (approximately 5 minutes)
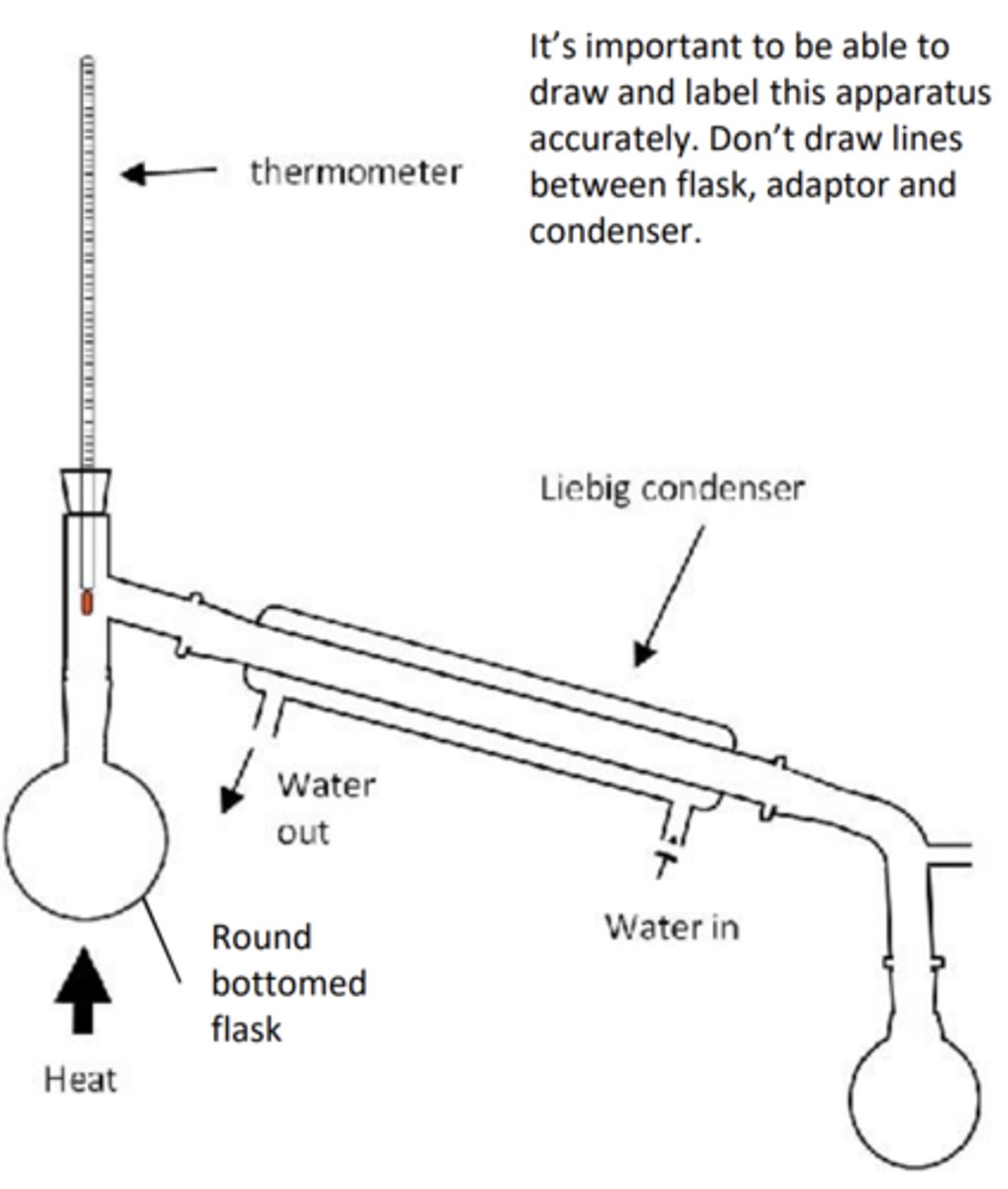
7. Position the flask in a hot water bath using water from a kettle. Light a Bunsen burner and maintain a boiling water bath for 20 minutes. Allow the apparatus to cool. 8. Distil your product using the apparatus shown and collect 3-4cm3 of clear, colourless liquid
Draw distillation apparatus
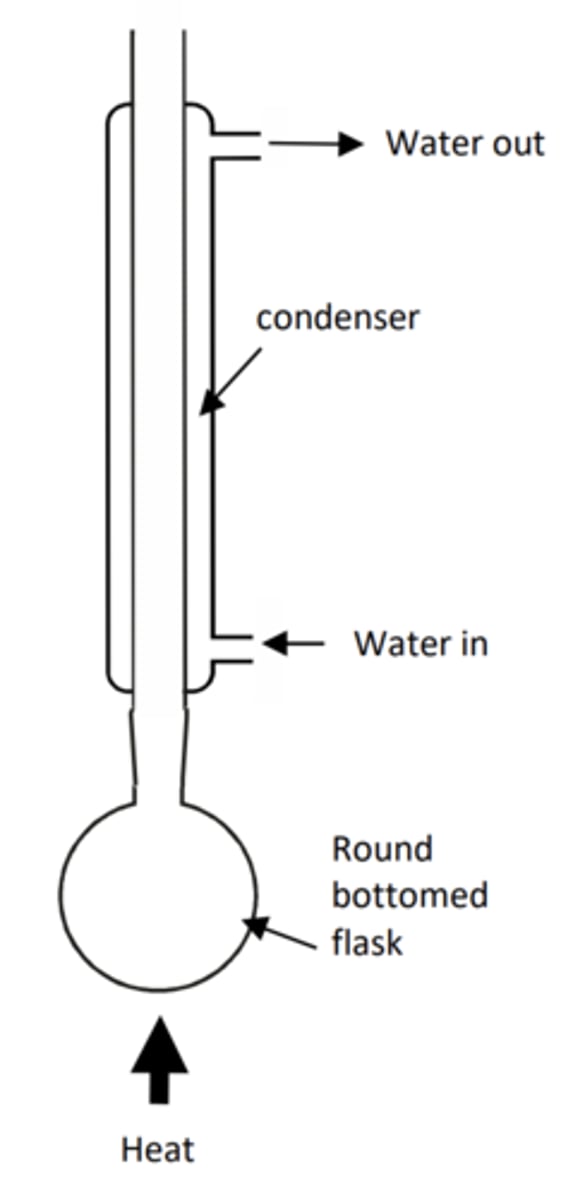
- Note the bulb of the thermometer should be at the T junction connecting to the condenser to measure the correct boiling point
- Note the water goes in the bottom of the condenser to go against gravity. This allows more efficient cooling and prevents backflow of water
What is the colour change of potassium dichromate?
Cr2O7 2- →→reduces to→→ Cr 3+
Orange → green
Where should clamps be secured?
To the joints, not near the middle as the glass here is the weakest and could shatter
Draw the apparatus for reflux
How would you carry out an experiment to investigate the chlorination of alcohol?
1. Add alcohol and conc HCl in conical flask and swirl, releasing the bung regularly as alcohol is volatile so might evaporate and build up gas pressure , for about 20 minutes
2. Add powdered anhydrous CaCl2 into the flask and swirl to make sure all the haloalkane is in the upper layer
3. Transfer to separating funnel and leave until mixture settles into 2 layers
4. Run off and discard the lower layer to discard leftover reactant
5. Add NaHCO3 solution to left over upper layer which reacts with the HCl
6. Shake the funnel and remove the bung afterwards, a few times since NaHCO3 + HCl → NaCl + H2O + CO2
7. Run off and discard the lower aqueous layer which contains the NaCl + H2O
8. Repeat the washing with NaHCO3 to make sure all HCl is gone
9. Run off the organic layer into a conical flask. Add a spatula of anhydrous Na2SO4 to remove the H2O, swirl to mix and leave until it looks completely clear
10. Decant into a round-bottomed flask and distil the solution, collecting the fraction that boils off between 50-52°C. Place this final product in a labelled sample tube
How is a flame test carried out?
1. Take a nichrome wire and clean by placing into a beaker of HCl and then place into a blue Bunsen flame
2. Repeat the cleaning until no colour change is produced
3. Dip the wire into the unknown metal compound and place into the flame
4. Observe the coloured flame
Give the expected results for flame tests?
Li: red, Na: orange/yellow, K: lilac, Rb: red, Cs: blue, Mg: no colour change, Ca: brick red, Sr: crimson, Ba: green
What is the test for NH4+?
1) Add NaOH and gently heat
2) If NH3 is given off NH4+ was present in reactants
NH4+ + OH- → NH3 + H2O
3) NH3 is alkaline therefore dissolves in H2O on damp red litmus paper turning it blue
eg. NH4Cl + NaOH → NH3 + H2O + NaCl
How do you test for SO42- and give the equation for this reaction
1. Add 3cm3 of acid eg. HCl to a test tube containing the compound
2. Add 3cm3 of barium chloride to the same test tube
Ba2+(aq) + SO42-(aq) → BaSO4(s) which is a white ppt.
Why would HCl be added before the previous reaction?
To remove carbonate ions as these could also produce a ppt and could be mistaken for BaSO4
Why can H2SO4 not be used instead of HCl?
Because it contains sulphate ions and so would give a false positive result
How do you test for halide ions?
1. Add 5cm3 of dilute nitric acid to the unknown halide ion in a test tube to remove ions that may interfere with the reaction
2. Add 3cm3 silver nitrate solution
3. Record the colour of the ppt. formed
What are the expected results for the halide ion test?
With silver nitrate:
AgCl: white ppt.
AgBr: cream ppt.
AgI: yellow ppt.
What is the role of nitric acid?
To react with any carbonates present to prevent formation of the precipitate Ag2CO3
How can the silver halide solutions produced be differentiated?
Add aqueous ammonia solution
AgCl: ppt dissolves and solution goes colourless
AgBr: ppt only dissolves in conc. ammonia to go colourless
AgI: does not dissolve even in conc. ammonia
How do you test for CO32- and HCO3- and give an equation for these reaction
1. Add 3cm3 of aqueous HCl to a test tube containing the compound
2. Bubble the gas evolved through limewater - will turn limewater cloudy
CO32-(s) + 2H+(aq) → CO2(aq) + H2O(l)
HCO3-(s) + H+(aq) → CO2(aq) + H2O(l)
How can you test for OH-?
Alkaline hydroxide ions will turn red litmus paper blue
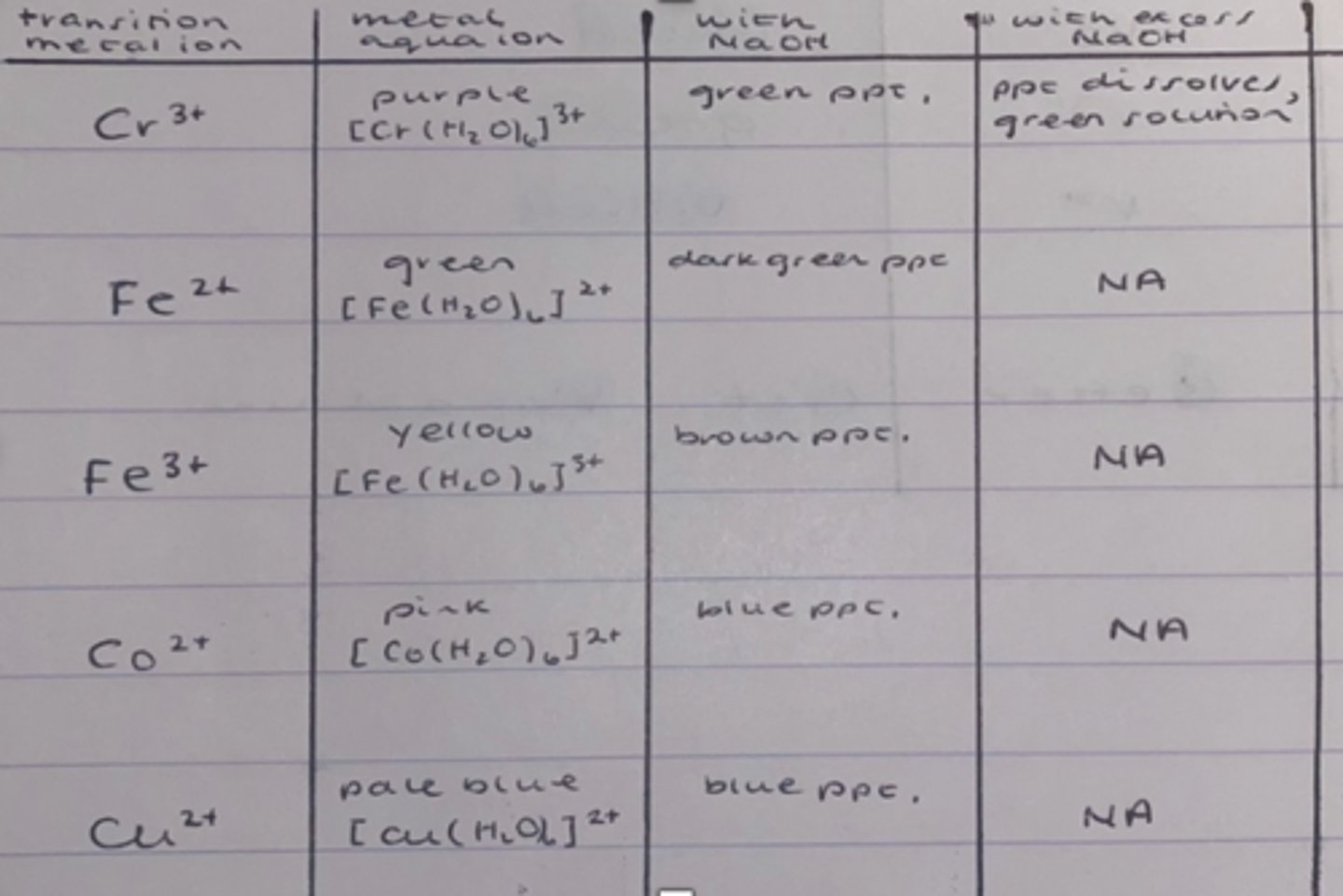
How do you test for a metal cation?
1. Dissolve the metal cation in distilled H2O to turn into a solution
2. Using a dropping pipette add 5 drops of sodium hydroxide
3. Note down the observation
4. Repeat this until there is no more change
What are the expected results when testing for a metal cation?
How do you identify an alkene?
1. Add 3cm3 of unknown substance to test tube
2. Add 3cm3 of aqueous bromine
3. Add a stopped and shake the test tube for 10 seconds
How do you identify an aldehyde?
1. 1cm3 of Fehling's 1 and 1cm3 Fehling's 2 to a test tube
2. Add 3cm3 of the unknown substance to the test tube
3. Swirl the test tube and place it in the water bath
How do you test for a carboxylic acid?
Write the equation for this reaction
1. Add 3g of sodium carbonate to the test tube containing the unknown compound
2. Bubble the gas evolved through limewater
2R-COOH + Na2CO3 → 2R-COONa + CO2 + H2O
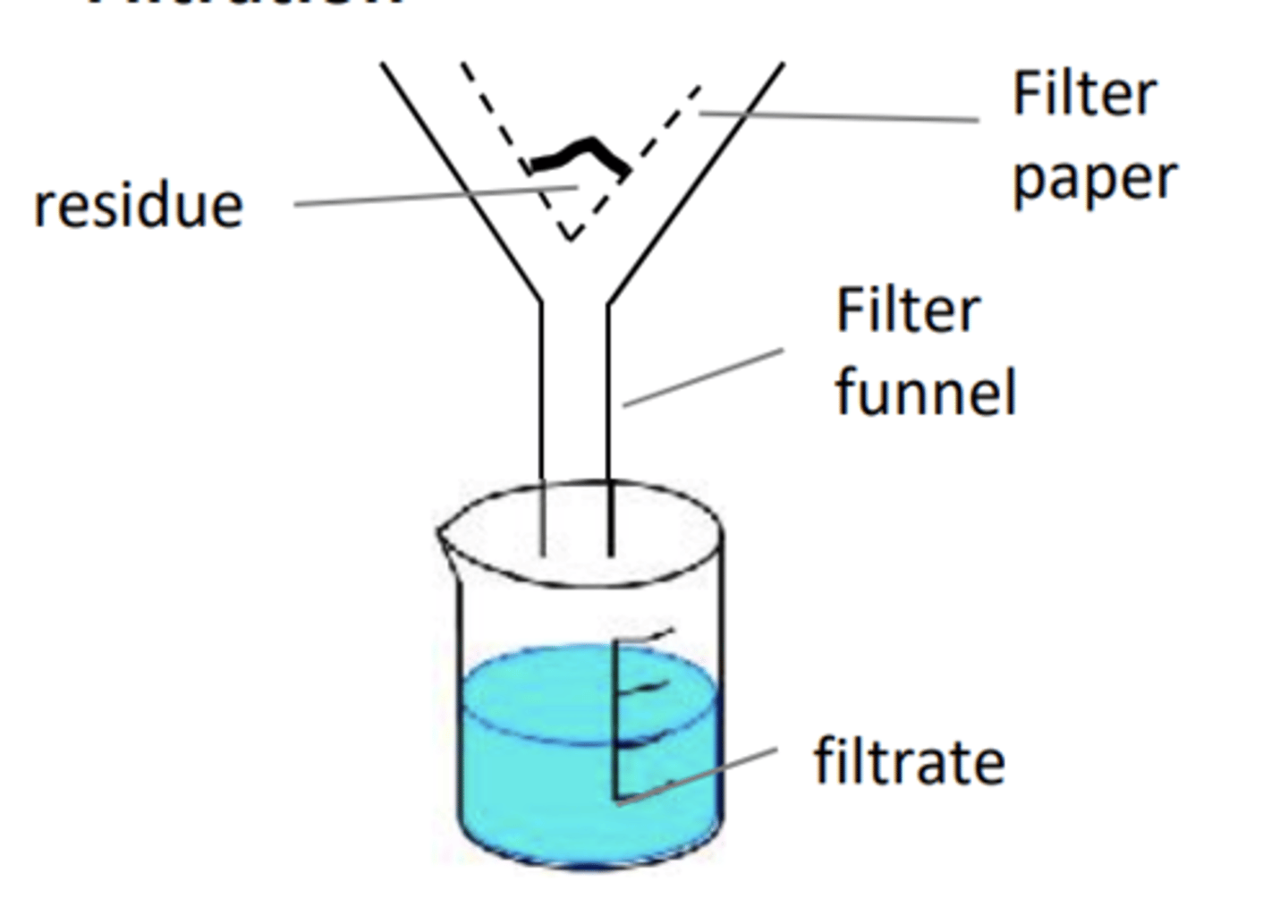
Draw gravity filtration
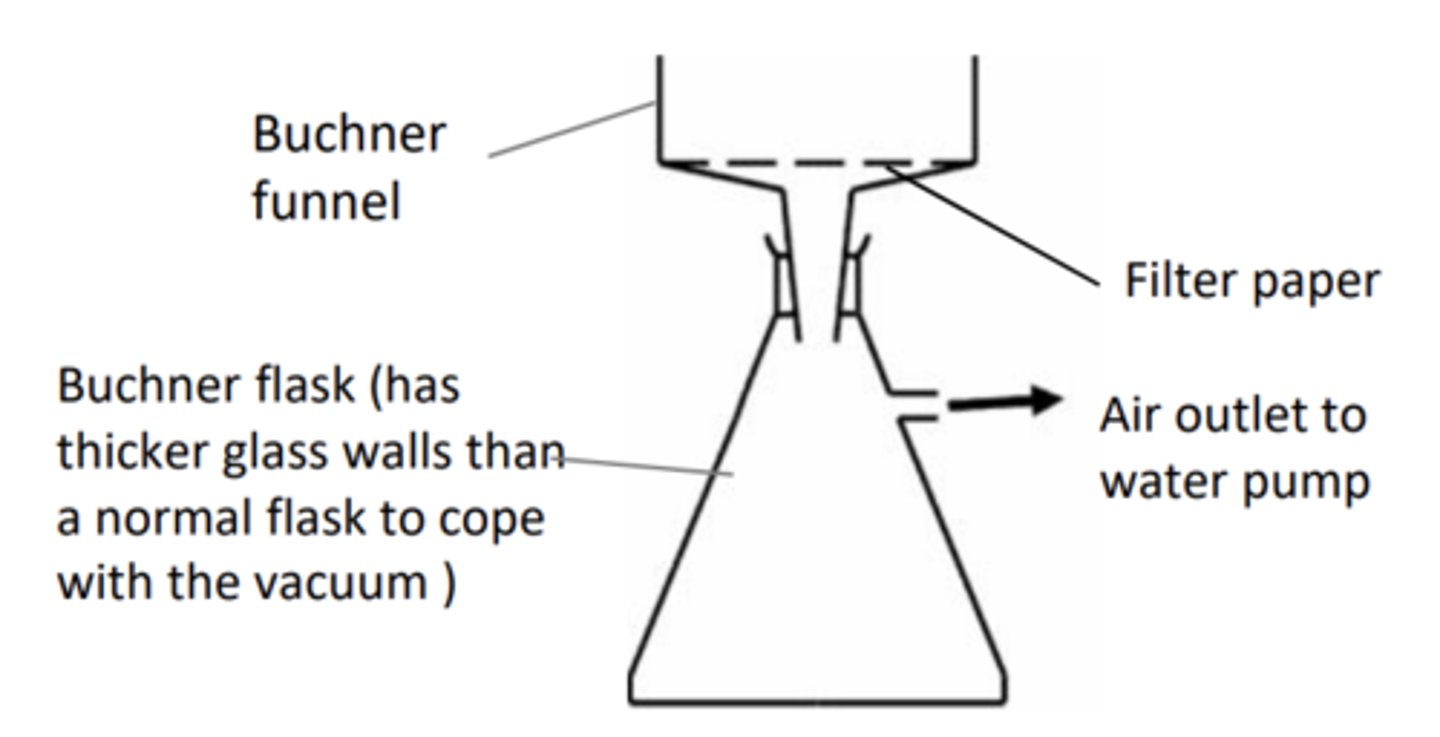
Draw vacuum filtration apparatus
What does 2,4-DNPH test for and what is a positive result?
Carbonyl group in aldehydes and ketones, orange ppt.
How can you differentiate between an aldehyde and a ketone?
Tollen's or Fehlings
How is Tollens carried out and what is a positive result?
1) Mix aqueous ammonia and silver nitrate
2) Heat gently with an aldehyde to oxidise it to carboxylic acid forming a silver mirror
3) Heat gently with a ketone, colourless solution as they cannot be oxidised
How is Fehling's carried out and what is a positive result?
1) Mix blue Cu2+ with sodium hydroxide
2) Heat gently with an aldehyde to oxidise it to carboxylic acid forming a brick red insoluble ppt.
3) Heat gently with a ketone, solution stays blue as cannot be oxidised
How is the iodoform test carried out and what is a positive result?
1) Mix iodine and sodium hydroxide
2) Warm gently with a methyl carbonyl to form a pale yellow ppt. (CHI3 - iodoform)
How would you carry out an experiment to find the rate equation for the reaction of iodine with propanone (titrimetric method)?
1) Add 25cm3 of propanone and 25cm3 of sulfuric acid in a conical flask
2) Start the stopwatch after adding 50cm3 of iodine solution, swirl conical flask
3) At equal intervals remove a sample of reaction using pipette and add to a conical flask
4) Add sodium hydrogen carbonate which stops the reaction noting time when the reaction is quenched
5) Titrate remaining iodine with sodium thiosulfate using starch indicator (colourless → black/blue)
What is the role of H2SO4 and sodium hydrogen carbonate?
Acid catalyst which is neutralised by quenching so the reaction is stopped
Give the reaction equation for the reaction in the conical falsk
CH3COCH3 (aq) + I2 (aq) → CH3COCH2 I(aq) + H+ (aq) + I- (aq)
Give the reaction equation of the titration
2S2O3 2-(aq) + I2 (aq) → 2I- (aq) + S4O6 2-(aq)
Why do concentrations of propanone and acid not change?
As they are in larger excess
How did the rate of change of iodine concentration change during the experiment?
I2 concentration is proportional to the volume of sodium thiosulfate added in the titration ∴ decreases as time goes on
↳ Measure of rate = volume of sodium thiosulfate/time taken
Explain the iodine clock reaction
I- + S2O8²- → 2SO4²- + I2
- Persulfate ions react with iodide ions to produce iodine
- By changing concentration of KI or sodium persulfate you change the rate of reaction
How would you carry out an experiment to determine a rate equation using a clock reaction?
1) In one beaker place known volumes of sodium persulfate solution and sodium thiosulfate solution, plus a few drops of starch solution
2) In another beaker place a known volume of potassium iodide solution
3) Mix the two, start a clock and stir. Stop the clock when the solution turns blue
4) Repeat the experiment with a different volume of sodium persulfate made up to the same total volume with water
5) Repeat the experiment with a different volume of potassium iodide solution, again making up to the same total volume with water
What is the purpose of sodium thiosulfate? Why is there a colour change in the iodine clock reaction?
a) So that the colour change is not too quick and it can be measured accurately
b) As the iodine is produced it reacts with the sodium thiosulfate, until all the sodium thiosulfate has been reacted
- The next iodine produced turns the starch from colourless to blue/black
How would you carry out an experiment to find the activation energy of a reaction?
1) Pipette phenol and Br solution into a boiling tube
2) Add 4 drops of methyl red indicator
3) Pipette H2SO4 into another boiling tube
4) Add boiling tubes to H2O bath at 15°, 25°, 35°, etc
5) When contents have reached temp of H2O bath add contents of one boiling tube into another then back into other boiling tube
6) Start stopwatch
7) Time how long until methyl red disappears as it is bleached by Br2 when all phenol has reacted
How would you carry out an experiment to find Ka of a weak acid?
1. Pipette 25.0 cm3 of 0.1 mol dm−3 ethanoic acid solution into a 250 cm3 conical flask
2. Measure initial pH of acid
3. Fill a burette with sodium hydroxide solution
4. Add two or three drops of phenolphthalein to the conical flask
5. Titrate the ethanoic acid with sodium hydroxide solution until the mixture just turns pink
6. Pipette a further 25.0 cm3 of 0.1 mol dm−3 ethanoic acid solution into the 250 cm3 conical flask
7. Record the pH of this solution
How do you calibrate the pH probe and why is this necessary?
- Measuring known pH of a buffer solution
- Necessary because pH meters can lose accuracy on storage
How do you improve the accuracy?
Keep a constant temperature
How do you find Ka in an experiment to find Ka of a weak acid?
Ka = [H+][CH3COO-]/[CH3COOH] however when a further 25cm3 of ethanoic acid is added [CH3COO-] and [CH3COOH] are equal so they cancel therefore...
Ka = [H+] and [H+] can be found in step 6 using a pH probe by doing [H+] = 10^-pH
How should organic substances be heated?
Using a water bath as they are flammable and could ignite if they were heated using a flame of a bunsen burner
How would you carry out an experiment to calculate the enthalpy change for the thermal decomposition of potassium hydrogencarbonate?
1) Place approximately 3g of solid K2CO3 into a test tube
2) Weight the test tube and its contents accurately
3) Use a burette to dispense 30cm3 of HCl into a polystyrene cup supported in a bealer
4) Clamp a thermometer into place making sure the thermometer bulb is immersed in solution
5) Measure the initial temperature of the acid
6) Continue measuring whilst the K2CO3 is added and record the highest temperature reached
7) Repeat using 3.5g KHCO3 and record the lowest temperature reached as this is exothermic
8) Use q=mcΔT to find enthalpy change
Why is it not possible to measure enthalpy change directly from the decomposition?
You have to take into account how much energy was put into the reaction in the first place
Write the symbol equation for the decomposition of K2CO3
K2CO3(s) → K2O(s) + CO2(g)
Write the symbol equation for the decomposition of KHCO3
KHCO3(s) → H2O(l) + CO2(g) + K2CO3(s)
What are some sources of error in an experiment to calculate the enthalpy change for the thermal decomposition of potassium hydrogencarbonate?
- Energy transfer from surroundings (usually loss)
- The method assumes all solutions have the heat capacity of water
- Neglecting the specific heat capacity of the calorimeter
- Ignore any energy absorbed by the apparatus
- Reaction or dissolving may be incomplete or slow
- Density of solution is taken to be the same as water
How can heat loss be reduced in an experiment to calculate the enthalpy change for the thermal decomposition of potassium hydrogencarbonate?
- Use a polystyrene cup
- Use a lid
- Place the cup in a beaker filled with cotton wool
How can calorimetry be carried out and what does it measure?
- Enthalpies of combustion can be calculated by using calorimetry
- Generally the fuel is burnt and the flame is used to heat up water in a metal cup
What needs to be measured for calorimetry?
- Mass of spirit burner before and after
- Temperature change of water
- Volume of water in cup
What are sources of error in calorimerty?
- Energy losses from calorimeter to surroundings
- Incomplete combustion of fuel
- Evaporation of fuel after weighing
- Heat capacity of calorimeter not included
- Measurements not carried out under standard conditions as H2O is gas, not liquid, in this experiment
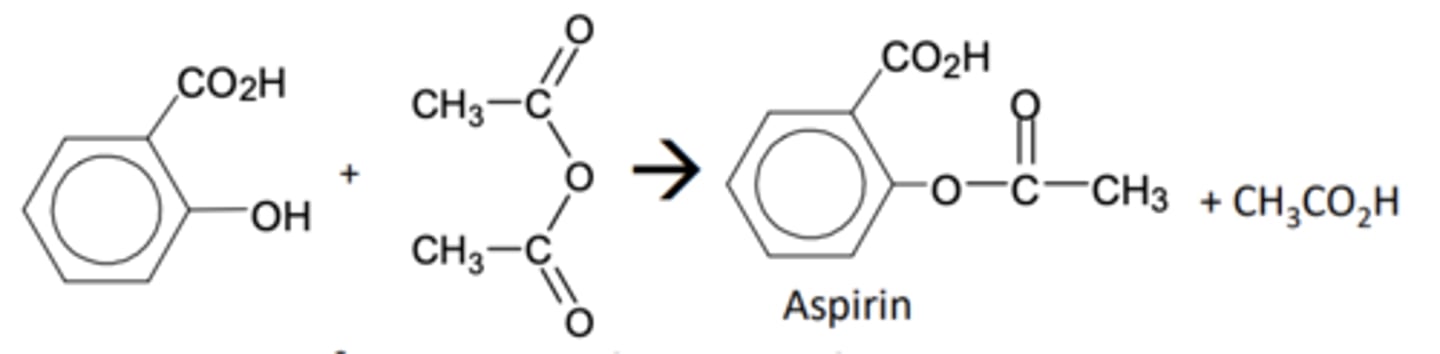
How would you carry out an experiment to synthesise aspirin from 2-hydroxybenzoic acid (part 1)?
1. Weigh 2.0 g of 2-hydroxybenzoic acid and put it in a pear-shaped flask. Clamp the flask and suspend it in a beaker of water
2. Add 5.0 cm3 of ethanoic anhydride to the 2-hydroxybenzoic acid
3. Add five drops of concentrated sulfuric acid to the mixture in the flask
4. Fix a condenser on the flask
5. In a fume cupboard, carefully warm the mixture in the water bath using a heating mantle
6. Gently swirl the mixture until all the solid has dissolved
7. Continue warming the mixture for another 10 minutes
8. Remove the flask from the hot water bath and add 10 cm3 of crushed ice and some distilled/deionised water and stir and rub the sides of the beaker with a stirring rod to induce crystallisation
9. Collect the product by suction filtration
How would you carry out an experiment to synthesise aspirin from 2-hydroxybenzoic acid (part 2)?
10. Wash the crystals with the minimum volume of hot ethanol
11. Pour the hot solution containing dissolved aspirin through a warmed filter funnel and fluted filter paper
12. Cool the filtrate by inserting beaker into ice
13. Suction filtrate with a Buchner flask to separate out crystals
14. Wash the crystals with distilled water to remove soluble impurities
15. Dry the crystals between absorbent paper
15. Measure the melting temperature of the aspirin using melting temperature apparatus
Describe the reaction when preparing asaprin?
Aspirin is made from 2-hydroxybenzoic acid which contains a phenol group
- The phenol group is turned into an ester by reacting it with the reactive ethanoic anhydride
What reactant can be used as an alternative to ethanoic anhydride but why is this not done?
An acyl chloride
- More expensive
- More corrosive
- More vulnerable to hydrolysis
- More dangerous
What is the purpose of the H2SO4?
Acts as a catalyst
Why do crystals form in step 8?
The cooler the temperature the lower the solubility so the greater the mass of crystals formed
Why could is be necessary to scratch with a glass rod to start crystallisation?
Crystals cannot form if no seed is present, once seed is present crystals will form rapidly
Why is there loss of yield?
- Crystals lost when filtering or washing
- Some product stays in solution after recrystallisation
- Other side reactions occurring
What effect do impurities have on the MP?
Melting point will be lowered and the sample will melt over a range of several degrees °C
How would you carry out an experiment to measure electrode potentials?
1) Clean the strips of zinc and copper using sandpaper
2) Degrease electrodes with cotton wool and propanone
3) Set up a zinc half-cell by pouring 50 cm3 of zinc sulfate solution into the 100 cm3 beaker and standing the strip of zinc in the beaker
4) Set up a copper half-cell by pouring 50 cm3 of copper(II) sulfate solution into a separate 100 cm3 beaker and standing the strip of copper in the beaker
5) Make an electrical connection with a strip of filter paper that has been dipped in a saturated solution of potassium nitrate
6) Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips
7) Record the electrode potential of the [Zn(s) | Zn2+(aq)] and [Cu2+(aq) | Cu(s)] system
8) If the voltmeter gives a negative value, reverse the connections so that it gives a positive value.
![<p>1) Clean the strips of zinc and copper using sandpaper</p><p>2) Degrease electrodes with cotton wool and propanone</p><p>3) Set up a zinc half-cell by pouring 50 cm3 of zinc sulfate solution into the 100 cm3 beaker and standing the strip of zinc in the beaker</p><p>4) Set up a copper half-cell by pouring 50 cm3 of copper(II) sulfate solution into a separate 100 cm3 beaker and standing the strip of copper in the beaker</p><p>5) Make an electrical connection with a strip of filter paper that has been dipped in a saturated solution of potassium nitrate</p><p>6) Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips</p><p>7) Record the electrode potential of the [Zn(s) | Zn2+(aq)] and [Cu2+(aq) | Cu(s)] system</p><p>8) If the voltmeter gives a negative value, reverse the connections so that it gives a positive value.</p>](https://knowt-user-attachments.s3.amazonaws.com/9bad2870-6b39-436a-8b4c-bb734d6f7f42.jpg)
What precautions should be considered when measuring electrode potentials?
- Some solutions are too dangerous to use at 1 mol dm-3 e.g. silver nitrate which is highly oxidising
- Zinc sulphate and iron (II) sulphate are harmful to the environment therefore have to be disposed of safely
Why are Zn and Cu used as electrodes?
As they are inert so don't react with water which would cause in increase the number of ions in solution so POE would be shifted to produce more metal atoms
What would differences between experimental values and theoretical values be due to?
The experiment was not carried out under standard conditions
How would you carry out an experiment to find the amount of iron in an iron tablet?
1) Crush 5 iron tablets with a pestle and mortar
2) Transfer to a weighing boat and weigh accurately after transferring to a small beaker
3) Add H2SO4 into the small beaker and stir to dissolve
4) Filter the solution to remove undissolved solid
5) Add to a volumetric flask and add all washings
6) Make up to mark with distilled water
7) Place the stopper in and shake
8) Pipette 25cm3 into the conical flask
9) Titrate with potassium manganate (VII) until mixture just turns pink
10) Repeat until you have concordant titres
Give the reaction equation
MnO4 -(aq) + 8H+ (aq) + 5Fe2+ (aq) → Mn2+ (aq) + 4H2O (l) + 5Fe3+ (aq)
How can thin-layer chromatography be used to identify amino acids?
1) Draw a pencil line near the bottom of the paper
2) Put a concentrated spot of each mixture on the baseline using a capillary tube
3) Place the paper in a beaker containing the solvent and cover with a lid or watch glass to prevent evaporation of the solvent
4) Different AA have different solubilities so move at different rates up the paper
5) Once solvent is near the top of the paper remove it from the solvent and immediately mark where the solvent reached
6) Some AA aren't visible so spray with ninhydride solution
7) Dry plate in oven
8) Work out Rf values = distance moved by spot/distance moved by solvent