AP Biology - Big Ideas from Unit #1: The Chemistry of Life
1/78
Earn XP
Description and Tags
Comprehensive Quizlet for AP Biology Unit #1: Chemistry of Life, primarily aligned to the 2020 CED (for the May 2025 exam) and also fully compatible with the 2025 CED (May 2026+ exams). 🎯 This set is designed as a review resource, not a replacement for instruction—it emphasizes core content, corrects common misconceptions, and integrates scenario-based practice aligned to AP Biology Science Practices (e.g., 1.C, 2.A, 6.E). ✅ Includes both fact recall and application-level flashcards to help build the thinking needed for a 4 or 5 on the exam. 🔁 Topics Covered: Water’s properties (Topic 1.1) Elements of life (1.2) Macromolecules: Carbs, Lipids, Nucleic Acids, Proteins (2020 1.3-1.6, 2025 1.3–1.7) Structure-function relationships Visual interpretation & predictive reasoning Ideal for daily review leading up to the AP exam.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
79 Terms
Water Properties
1) Cohesion
2) Adhesion
3) High Surface Tension
4) High Specific Heat (High Heat Capacity)
5) Great solvent for life! (Closest thing we have to a universal solvent)
6) Less dense as a solid
Hydrogen Bond
An intermolecular bond between 1 Hydrogen Atom (on one molecule) and a slightly electronegative atom (on the other molecule).
Hydrogen atoms usually bond with one of the FON.
F - Fluorine
O - Oxygen
N - Nitrogen
Hydrogen Bonds in Water
They are located between the slightly electropositive Hydrogen atom of one molecule and another molecule's slightly electronegative oxygen atom.
Hydrogen Bonds in DNA
They are located between nitrogenous bases. The DNA double-helix structure is held together by a phosphodiester covalent bond, but the nitrogenous bases are held together by hydrogen bonds.
IDENTIFY and EXPLAIN the effect of ionic compounds (ex. Salt - NaCl) on hydrogen bonding between water molecules.
Ionic compounds (e.g., NaCl) dissociate in water into Na⁺ and Cl⁻ ions. These charged particles interfere with hydrogen bonds between water molecules by attracting the partial charges on water’s hydrogen and oxygen atoms. This weakens cohesion and can affect: Protein folding (denaturation), Membrane behavior, Solubility of biomolecules.
Salt ions can disrupt structure and function at the molecular level — a key concept in protein chemistry and cell physiology.
THINK: “Salt steals friends.” Sodium and chloride steal water molecules away from bonding with each other — just like a third wheel disrupting a strong friendship. That’s why hydrogen bonds break and structures fall apart.
Polar molecule
Uneven distribution of electric charge (+/-)
T/F: Water is a polar molecule.
True! Water is a polar molecule due to the uneven distribution of charge between the electronegative oxygen atom and the electropositive hydrogen atom.
What do the properties of water result from?
They result from the polarity of the water molecule and hydrogen bonding between water molecules.
Cohesion
Property of water - Attraction between water molecules - Water sticks together!
Adhesion
Property of water - Attraction between water molecules and polar substances. Water sticks to other polar substances!
Why can water move up a xylem (capillary action)?
Water can move up a xylem, from the roots to the leaves in a plant, due to its properties of cohesion and adhesion, as cohesion keeps the water molecules together and adhesion keeps water attached to the xylem of the plant (polar!).
Water is held together by a ___________________________________.
Water is held together by a polar covalent bond!
High Surface Tension
Property of water - Difficulty in breaking through the liquid surface of water due to Hydrogen bonds above and below.
*Connected to Cohesion
High Specific Heat & Ability to Moderate Temperature
Water property - Water is an effective heat bank due to hydrogen bonding as when hydrogen bonds are broken, thermal energy (heat) is absorbed and when bonds are formed, thermal energy is released. Since water's able to absorbe thermal energy w/o having a drastic change in its own temperature, it has an ability to moderate temperature.
Ex. Temperatues in Bozeman, MT vs Seattle, WA.
Evaporative Cooling
Water property - As water (liquid) evaporates, the surfaceof the liquid that remains cools down.
Think: Sweat.
Good Solvent for Life - Closest thing to a universal solvent
Water property - Water is the closest thing we have to a universal solvent since it can dissolve all polar molecules.
"Like disolves like"
Hydrophillic
Affinity/Attraction for Water - Water-LOVING!
All polar molecules are hydrophilic.
Hydrophobic
NO affinity/attraction for Water - Water-HATING!
All nonpolar molecules are hydrophobic.
Less Dense as a Solid
Water property - Water is less dense as a solid! ICE FLOATS! Water forms crystal-like bonds when it freezes, putting the molecules at fixed distances from each other.
Why it matters: Floating ice allows for life to exist overwinter in places like lakes. As it is, the floating ice insulates the water below so life can continue until spring thaw. Living systems depend on the properties of water that result from its polarity and hydrogen bonding.
Elements of Life
Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus, Sulfur
CHONPS!
Elements found in Carbohydrates
CHO
Note: Carbohydrates always have a CHO ratio of 1:2:1.
Ex: C₆H₁₂O₆ (Glucose!)
Elements found in Lipids
CHO
Note: Phospholipids have CHO + P!
Elements found in Proteins
CHON + S*
Note: Sulfur is sometimes found in proteins. Most of the time, it's just CHON.
Elements found in Nucleic Acids
CHONP
Biological Macromolecules
Carbohydrates, Lipids, Proteins, Nucleic acids
CHO - Carbs
CHO - Lipids
CHON + S* - Proteins
CHONP - Nucleic Acids
Functions of Carbohydrates in Living Organisms
1) Provide Energy
2) Store Energy
3) Build Macromolecules
Functions of Lipids in Living Organisms
1) Important constituent for cell membranes
2) Building blocks of many hormones
3) Cells store energy for long-term usage in the form of lipids (fats)
Functions of Proteins in Living Organisms
1) Catalyze reactions (enzymes)
2) Provide structural support
3) Provide defenses
Functions of Nucleic Acids
1) Store genetic information
2) Guide protein synthesis
3) Facilitate appropriate linkage of polypeptides
Glycosidic Bond
covalent bond between two monosaccharides
Peptide Bond
covalent bond formed between amino acids
Phosphodiester Bond
covalent bond that is responsible for the polymerization of nucleic acids by linking sugars and phosphates of adjacent nucleotides
Dehydration Synthesis
To build polymers from monomers while losing water.
Hydrolysis
To split a polymer into monomers while gaining water.
Describe the properties of the monomers and the type of bonds that connect the monomers in biological macromolecules.
Hydrolysis and dehydration synthesis are used to cleave and form covalent bonds between monomers.
How does the R group affect protein folding?
It affects tertiary and quarternary structure(s), altering the 3D shape.
Why does structure determine function in macromolecules?
The 3D structure of macromolecules (especially proteins) determines how they interact with other molecules and carry out their functions in cells.
Changes in structure = changes in function.
These theme is common in AP Biology and the general study of Biology.
pH
Measure of Acidic/Alkaline (Basic) a solution is.
pH and H+ ion concentration have a _______ relatonship
pH and H+ ion concentration have an INVERSE relatonship.
⬆️ H+ Concentration = ⬇️ pH (more acidic)
Acidic pH
pH less than 7 is ACIDIC!
Alkaline (Basic) pH
pH greater than 7 is ALKALINE (BASIC)!
Neutral pH
pH = 7
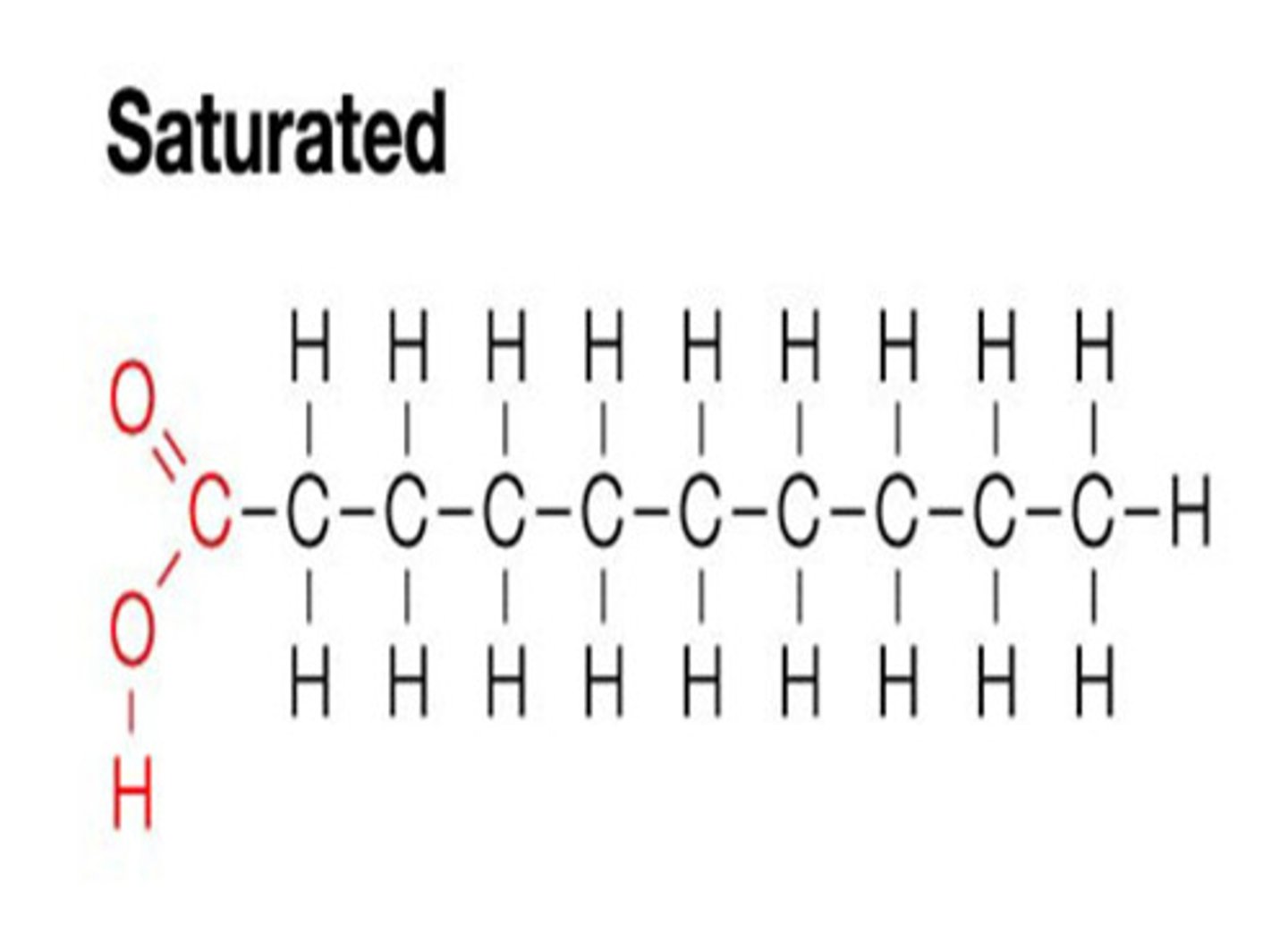
Saturated Lipid
Structure: Contain only single bonds between carbon atoms in the fatty acid chains.
Kinks?: No kinks - the hydrocarbon chains are straight.
Packing: Because of their straight structure, they pack tightly together.
State at Room Temp: Typically solid (e.g., butter).
Sources: Commonly found in animal fats.
Saturated with Hydrogen Atoms! NO DOUBLE BONDS
Unsaturated Lipid
Structure: Contain one or more double bonds between carbon atoms.
Kinks?: Yes, the double bonds create kinks or bends in the fatty acid chains.
Packing: These kinks prevent tight packing, so they are less dense.
State at Room Temp: Typically liquid (e.g., olive oil).
Sources: Commonly found in plant oils and fish.
DOUBLE BONDS PRESENT!
Monomer of Carbohydrate
monosaccharide
Monomer of Protein
Amino Acid
Components of Amino Acid: (Amine Group on left, ⍺-carbon in center, R group in center top, Hydrogen atom in center bottom, Carboxyl group on right)
Monomer of Nucleic Acid
Nucleotide
Components of Nucleotide: (Pentose Sugar, Charged Phosphate Group, Nitrogenous Base)
Components of a Phospholipid
hydrophilic head and hydrophobic tail
Which can be broken down by animals: Starch or Cellulose?
Starch
Protein Structure
a) Primary Structure = Poly-peptide chain, peptide covalent bonds involved
b) Secondary Structure = Hydrogen bonds between amino acids, no R-group action, proteins folded into the a-helix or b-pleated sheet. No R-group action.
c) Tertiary Structure = Bonds between R-groups such as hydrogen bonds, ionic bonds, hydrophobic interactions, disulfide bridges. The protein forms its 3D shape.
d) Quarternary Structure = Tertiary Structure but with Multiple Polypeptide Chains
Primary Structure
Poly-peptide chain, peptide covalent bonds involved
Secondary Structure
Hydrogen bonds between amino acids, no R-group action, proteins folded into the α-helix or β-pleated sheet. No R-group action.
Tertiary Structure
Bonds between R-groups such as hydrogen bonds, ionic bonds, hydrophobic interactions, disulfide bridges. The protein forms its 3D shape.
Quarternary Structure
Tertiary Structure but with Multiple Polypeptide Chains
Why is the specific order of amino acids in a polypeptide (primary structure) so important?
In proteins, the specific order of amino acids in a polypeptide (primary structure) determines the overall shape of the protein. Order of amino acids in a polypeptide determined by DNA.
R groups
Groups that give different amino acids different properties. Variable groups. Interactions between R groups are very important regarding Tertiary and Quaternary Structure!
Are lipids polar or nonpolar?
Lipids are nonpolar molecules that do not follow the standard "monomer-polymer" rule.
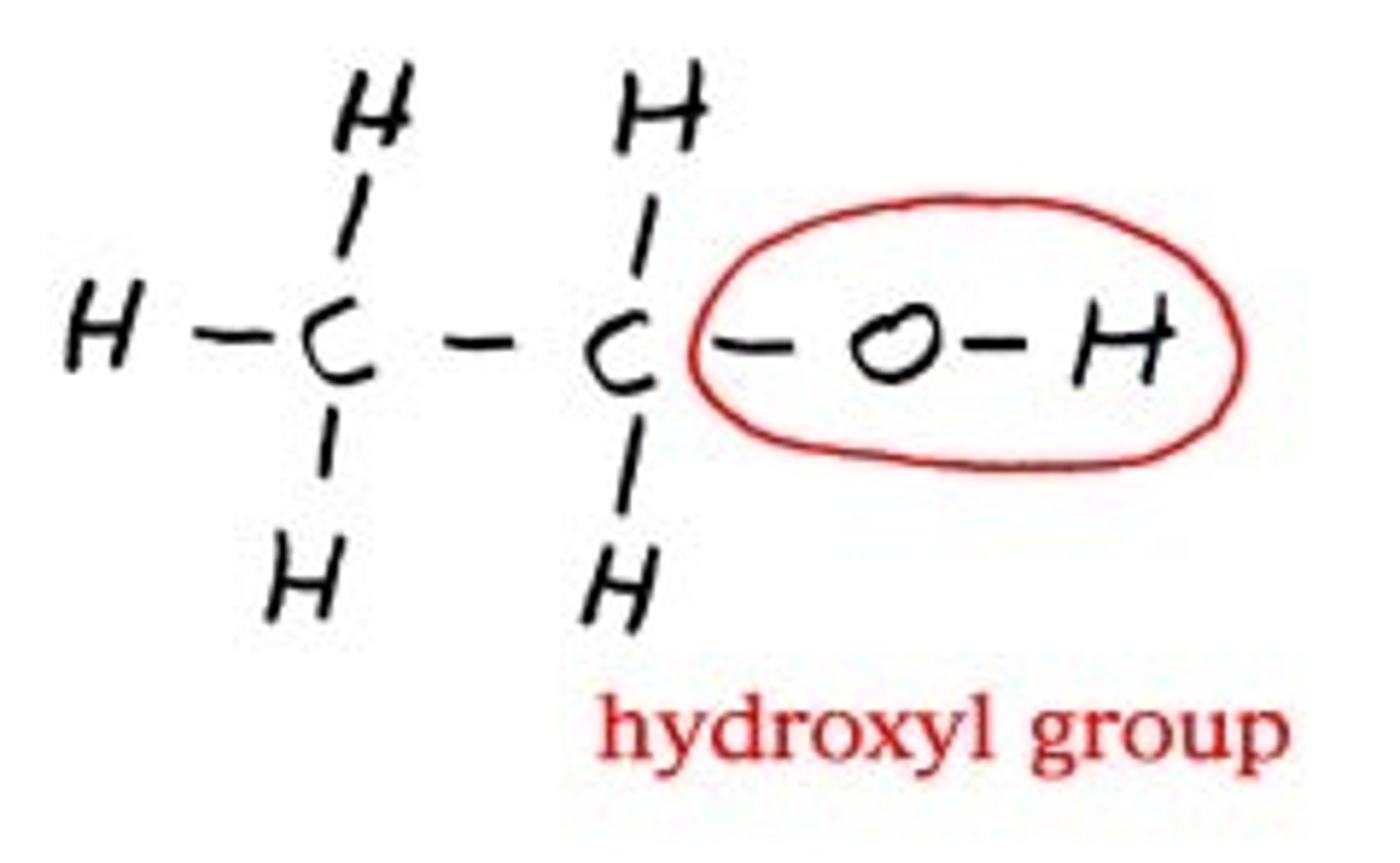
Hydroxyl Group (-OH)
A polar functional group is found in alcohols and carbohydrates. Increases solubility in water by forming hydrogen bonds.
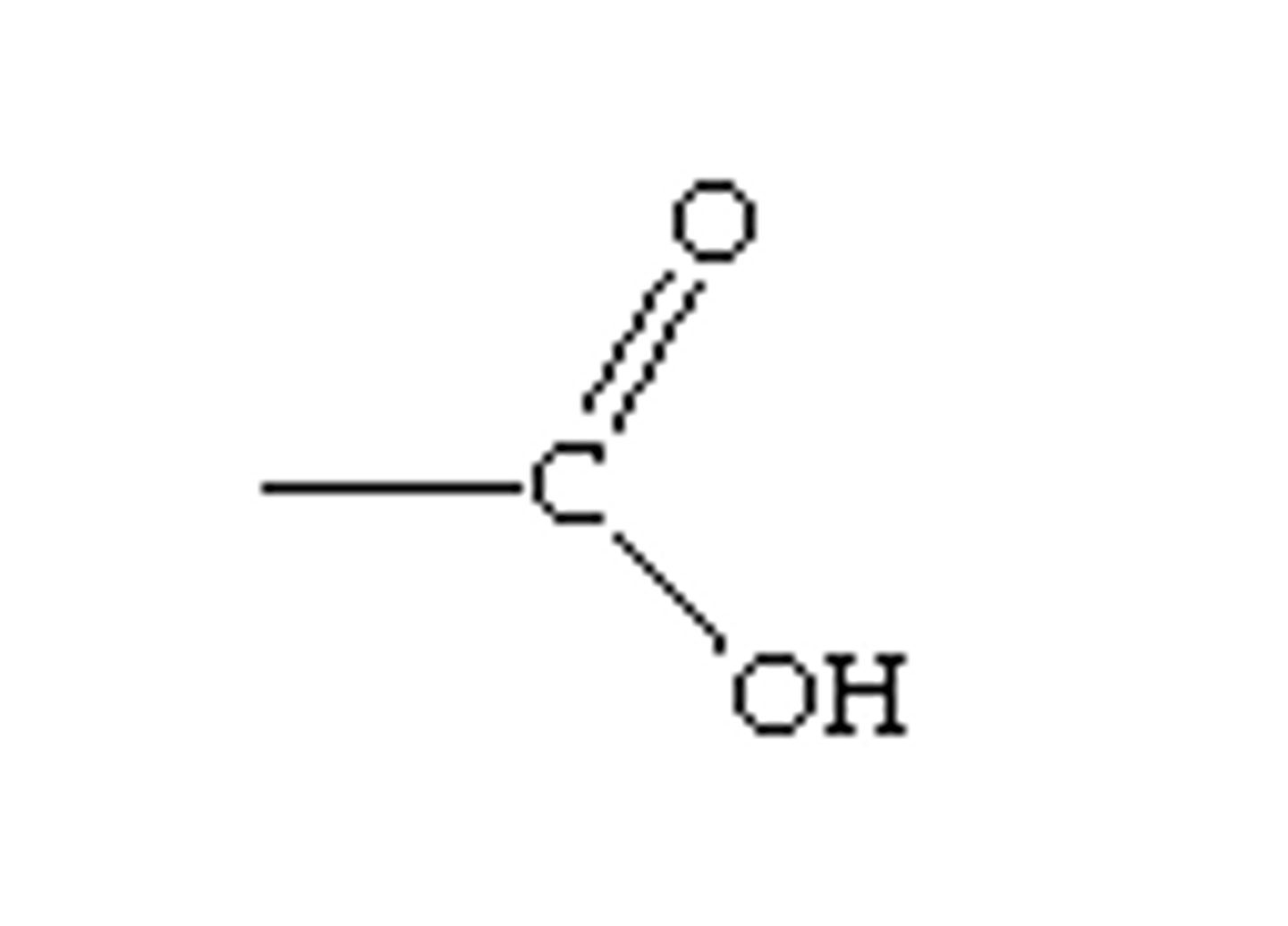
Carboxyl Group (-COOH)
Acts as an acid by donating an H⁺ ion. Found in amino acids and fatty acids. Contributes to acidic properties in molecules.
Amino Group (-NH₂)
Acts as a base by accepting an H⁺ ion. Found in amino acids. Plays a role in peptide bond formation during protein synthesis.
It is part of the AMINO ACID STRUCTURE!
Phosphate Group (-PO₄²⁻)
Adds a negative charge to molecules and is involved in energy transfer (like in ATP). Found in nucleic acids and phospholipids. Highly polar and reactive.
*DNA is slightly electronegative due to the charge in the phosphate group. This is important to know for 6.8 Biotechnology, such as DNA Gel Electrophoresis (Unit #6).
Sulfhydryl Group (-SH)
Found in the amino acid cysteine. Forms disulfide bridges that help stabilize the tertiary structure of proteins.
Methyl Group (-CH₃)
A nonpolar and hydrophobic functional group. Often involved in gene regulation, such as DNA methylation, which can silence gene expression. Common in lipids and epigenetic modifications.
*This ties into Unit #6 when we discuss Epigenetics, specifically DNA methylation.
Skill/Scenario Practice: What happens to a protein if a hydrophobic R-group is replaced with a hydrophilic one?
It could misfold because the hydrophilic R-group may interact with water instead of staying in the protein’s hydrophobic core, disrupting the tertiary structure and function. The structure would be altered, meaning there would be a change in function.
Science Practice: 6.E - Argumentation & Prediction
Why It Matters: This kind of mutation-based reasoning appears in FRQs and MCQs, testing how changes in structure affect function.
Skill/Scenario Practice: A mutation causes a polar amino acid to be replaced by a nonpolar one. Predict the effect on protein folding.
This may cause misfolding, especially if the region is normally exposed to water, disrupting tertiary or quaternary structure. Once again, there would be a change in the protein folding and function as the structure has been altered.
Science Practice: 6.E - Argumentation & Prediction
Why It Matters: You’ll often need to justify the impact of chemical changes on protein function, especially in system-focused questions.
Skill/Scenario Practice: Why do phospholipids form bilayers in water?
Their hydrophilic heads interact with water, while hydrophobic tails cluster inward, forming a bilayer.
Science Practice: 1.C – Concept Explanation
Why It Matters: This concept shows up frequently in early FRQs, membrane-based questions, and MCQs.
Skill/Scenario Practice: How does the sequence of amino acids in a protein (primary structure) determine its final 3D shape?
The sequence determines how R-groups interact (hydrophobic, ionic, disulfide bonds), which drives tertiary and quaternary structure.
Science Practice: 1.C – Concept Explanation
Why It Matters: College Board loves asking you to connect sequence → structure → function.
Skill/Scenario Practice: In a diagram of three water molecules, where are the hydrogen bonds located?
Between the partially negative oxygen of one molecule and the partially positive hydrogen of another.
Science Practice: 2.A - Visual Representations
Why It Matters: These diagrams appear in MCQs and lab FRQs. You must identify molecular interactions quickly.
Skill/Scenario Practice: A structural diagram shows a fatty acid with kinks. What type of fat is it?
An unsaturated fat; the kinks are caused by double bonds in the hydrocarbon chains. Recall that fats are lipids!
Science Practice: 2.A - Visual Representations
Why It Matters: You’ll need to distinguish saturated vs. unsaturated fats visually and understand how this affects fluidity.
Skill/Scenario Practice: A macromolecule contains nitrogenous bases, phosphate groups, and a sugar. What is it?
A nucleic acid (either DNA or RNA) as only nucleic acids contain CHONP.
Science Practice: 1.A – Concept Identification
Why It Matters: You’re expected to ID macromolecules from structure and function. This shows up in experimental setups and basic MCQs.
Skill/Scenario Practice: A molecule has long hydrocarbon chains, no nitrogen, and is nonpolar. What macromolecule is it?
A lipid.
Science Practice: 1.A – Concept Identification (focusing on Macromolecule Identification - 2020 CED Topics 1.3-1.6, 2025 CED Topics 1.3-1.7)
Why It Matters: Lipids are structurally unique; you’ll be asked to recognize them and understand their hydrophobic behavior
Skill/Scenario Practice: In an experiment, a student finds that water rises up a thin capillary tube. Which property of water explains this?
Adhesion and cohesion via hydrogen bonding.
Science Practice: 4.B – Data Interpretation
Why It Matters: You must interpret phenomena like transpiration using water’s properties.
Skill/Scenario Practice: A graph shows protein denaturation above 60°C. Why?
Heat disrupts hydrogen bonds and other interactions that stabilize protein structure.
Science Practice: 4.B - Data Interpretation
Why It Matters: Experimental data questions often ask you to explain a graph using molecular reasoning.
Skill/Scenario Practice: T/F - Lipids are made of repeating monomers.
FALSE! Lipids are not true polymers as they don’t have repeating monomer units.
Science Practice: 1.A – Concept Identification
Why It Matters: Commonly tested trick question—avoid the trap!
Skill/Scenario Practice: T/F - Proteins are made of nucleotides.
FALSE! Proteins are made of amino acids; nucleotides are monomers of nucleic acids (DNA/RNA).
Science Practice: 1.A – Concept Identification
Why It Matters: This misconception appears often in MCQs
Skill/Scenario Practice: T/F - A phospholipid is entirely hydrophobic.
FALSE! It has a hydrophilic phosphate head and hydrophobic fatty acid tails.
Science Practice: 1.C - Concept Explanation
Why It Matters: Amphipathic nature of phospholipids is crucial for understanding the cell membrane. *Connects to Unit #2.
Skill/Scenario Practice: If an enzyme's active site becomes nonpolar instead of polar, how will substrate binding be affected?
Polar substrates will not bind well, reducing enzyme activity. Due to the change in structure of the enzyme, there will be a change in function of the enzyme. Thus, polar substrates will not be able to bind well to the enzyme as it has a nonpolar active site, meaning only nonpolar substrates will be able to bind well to the active site.
Science Practice: 6.E - Argumentation & Prediction & 1.C - Concept Explanation
*This connects to Unit #3, when we're discussing Enzymes and Changes in Enzyme Structure & Function.
Why It Matters: This bridges Unit 1 & 3. AP loves testing structure-function through real molecular examples.
Skill/Scenario Practice: Differentiate between a protein and a proton.
A protein is a large, complex molecule made up of amino acids that perform various functions in many organisms, while a proton is a subatomic particle with a positive charge found in the nucleus of an atom.
Protein = Biological Macromolecule that plays crucial functions such as catalyzing reactions (enzymes!), providing structural support, and defense.
Proton = Subatomic particle with a positive charge found in an atom’s nucleus.
Science Practice: 1.A - Concept Explanation
Why It Matters: Popular misconception previous students have made during the AP Biology EXAM.
Skill/Scenario Practice: A researcher introduces a mutation into a gene that prevents part of the resulting polypeptide from forming an alpha-helix during protein folding. Identify which level(s) of protein structure would be affected by this mutation, and explain why.
Primary structure would not be affected because the amino acid sequence remains unchanged.
Secondary structure would be affected because the alpha-helix is a type of secondary structure, and it cannot form correctly.
Tertiary structure would be affected because the overall 3D folding of the protein depends on proper secondary structure.
Quaternary structure would be affected if the protein normally interacts with other subunits, because misfolded tertiary structures prevent correct quaternary assembly.
Science Practice: 1.C - Applied Concept Explanation
Why It Matters: Being able to explain changes in protein structure and how it impacts the protein is a commonly tested question type for Unit #1 on the AP Exam.