E L2 - Hormone Action (cell surface receptors)
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Learning Objectives
principles of hormone-receptors interaction
pathways utilised by cell surface hormone receptors
importance of hormone/receptor interactions
hormone families
hormones = chemical messengers
peptide - water soluble + can be stored
steroid - similar to lipids
amino acid derivatives
hormones interact with receptors to induce responses within cells
hormones alter protein function or gene transcription
hormone binds to receptor = either slow / fast response
fast = altered protein function
taking something already there and modifying it
sec/mins
slow = altered gene expression
sometimes involves upregulation of a gene + then transcribed into something
mins/hours
alters cell behaviour
hormones alter protein function or gene transcription (image)
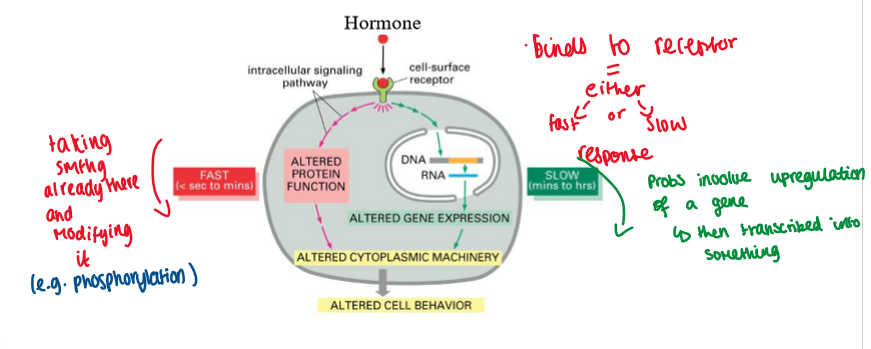
what is a hormone receptor?
bind hormone specifically and be able to detect it among other related molecules
bind the hormone with high enough affinity = to detect the hormone in the blood
the receptor must be only on specific tissues (so only those respond to hormone)
the receptor must be saturable + there must be a limited number of binding sites (to control strength of reaction)
how to turn off response - it must be reversible (a way to remove hormone)
types of hormone receptors
cell surface receptors
intracellular receptors
cell surface receptors - linked to TK
mainly for peptide hormones bc they cannot pass through the cell membrane (water soluble)
these receptors rely on phosphorylation
kinase = usually means it phosphorylates something
cell surface receptors diagram
(hormone receptor) cell surface receptors - linked to TK (tyrosine kinase):
either
growth factor receptors intrinsic TK (e.g. insulin)
cytokine receptors recruit TK (e.g. prolactin)
intrinsic or recruited TK = end is the same
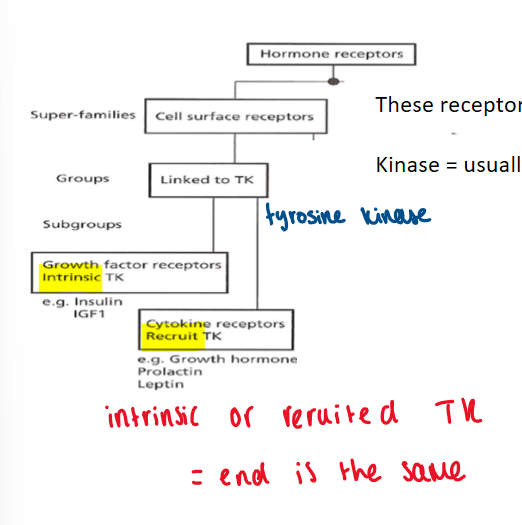
tyrosine kinase
an enzyme that transfers a phosphate group from ATP to to a tyrosine residue in a protein
phosphorylation indices conformational changes
tyrosine kinase activity can be either - intrinsic or recruited
intrinsic tyrosine kinase activity: examples
Epidermal Growth Factor receptor (EGF)
insulin
intrinsic tyrosine kinase activity: EGF as an extracellular receptor
dont need to learn all this j an example
EGF family of receptors (EGF 1-4)
ligand-induced dimerisation
peptide ligands (encoded by specific genes) - cleaved to yield active hormone
autocrine, paracrine cell signalling
signal transduction processes
Ras
phosphatidylinositide 3-kinase (PI 3-kinase)
JAK-STAT
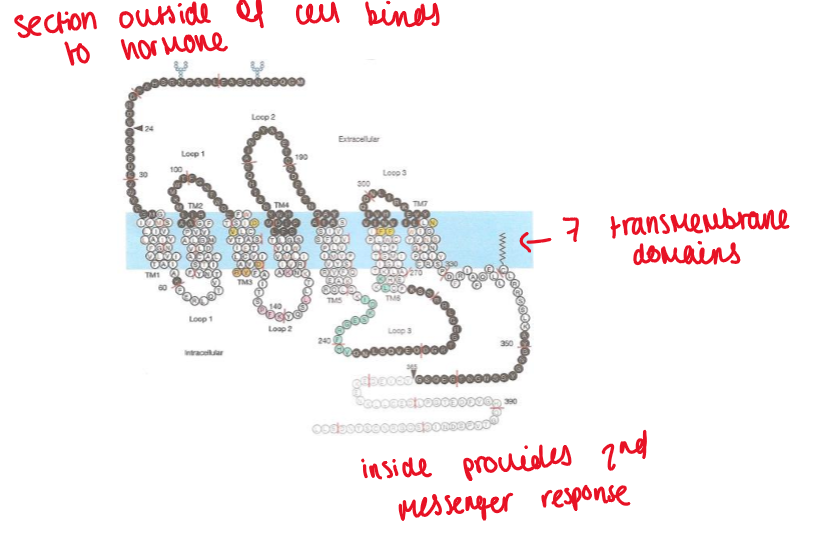
EGF as an extracellular receptor
membrane receptors = structured molecules that cross the outer cell membrane
the EGF receptor has:
a hormone binding site
2 cysteine-rich regions
a single trans-membrane region
a kinase domain (will become activated once hormone binds
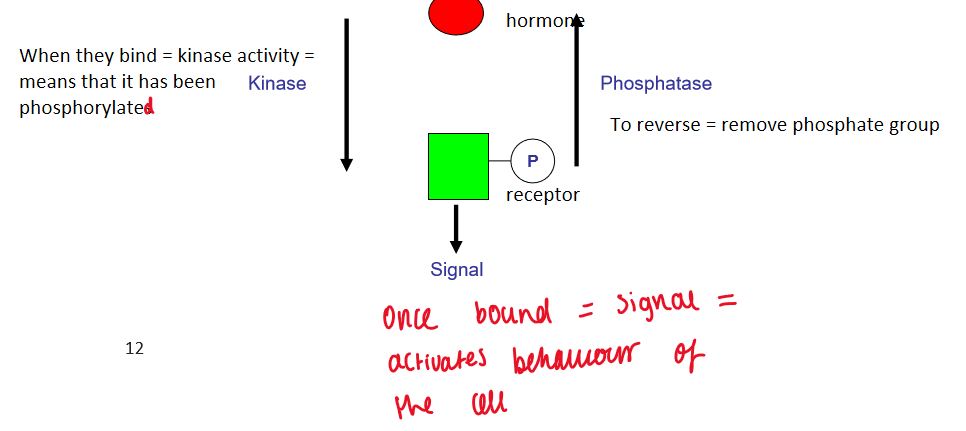
Basic mechanism of receptor modification
post-translational modification
when they bind = kinase activity = means it has been phosphorylated
to reverse - phosphatase (removes phosphate group)
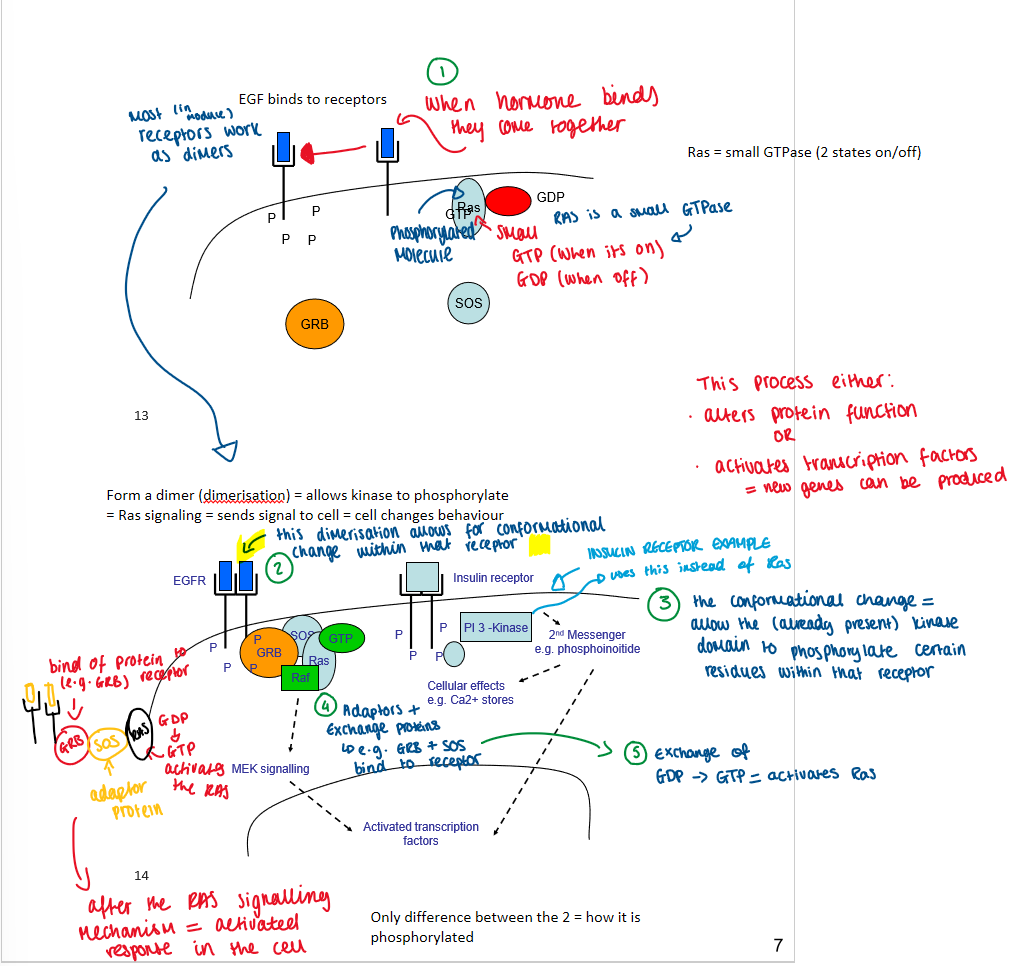
EGF + intrinsic TK
EGF binds to receptors
receptors bind to form a dimer (dimerisation)
dimerisation allows for conformational change within receptor
the conformational change = allows the (already present) kinase domain to phosphorylate tyrosine residues on receptor
this allows adaptor proteins to bind
this is where GDP (inactive) changes to GTP (active) = activates Ras signalling cascade = change in cell behaviour
active Ras-GTP triggers
same occurs for insulin but with PI 3-kinase instead of Ras
only difference between the two is how its phosphorylated
EGF + intrinsic TK (diagram)
recruited tyrosine kinase activity
these receptors do not have an intrinsic kinase domain (outsider does it - JAK)
JAK STAT - protein (Janus Associated Kinase Signal Transduction and Transcription)
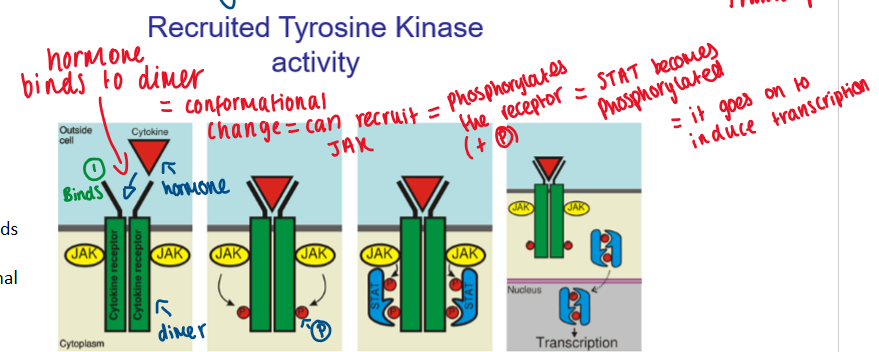
hormone binds to dimer = conformational change - can recruit JAK
JAL phosphorylates the receptor
STAT becomes phosphorylated
allows it to go on and induce transcription
end point is same = cell activated
recruited tyrosine kinase activity diagram
cell surface receptors - linked to G proteins
G-protein coupled receptors
rely on second messenger
cell surface receptor doesn’t have access to inside
its like a relay, passing baton - passes from receptor to signalling pathway
G protein coupled receptor
G protein has 2 states:
bound to GTP = active
bound to GDP = inactive
act via 2nd messenger molecules to transfer signal to cell:
second messengers: cyclic AMP, inositol 1,4,5-triphosphate (IP3), Diacylglycerol (DAG)
phosphorylation and calcium flux are important features of signal transduction
G Subunits
G proteins are heterotrimeric
a, b and y subunits
b/y subunits = for a single functional unit
our focus - alpha subunit
many Isoforms of a-subunits:
4 subfamilies (Gsa, Gia, Gqa, Goa)
activation of receptor releases a subunit
G coupled receptor signalling
resting G protein a-subunit associated with GDP
activation of receptor by hormone induces conformational change to receptor
induces conformational change to a-subunit = allows exchange of GDP to GTP (GDP = D = DEAD)
a-subunit is release + activates 2nd messenger
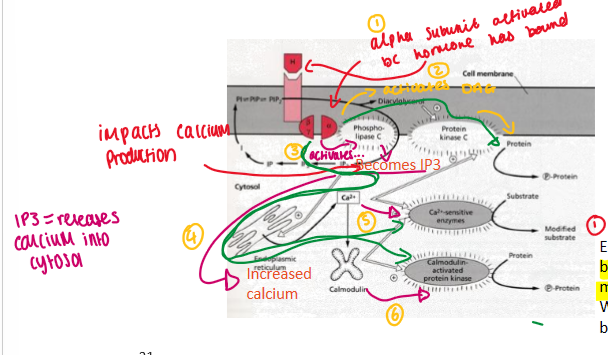
Diaglycerol (DAG) and Ca2+
alpha subunit activates bc hormone has bound
activates DAG
activates phospho-lipase C = becomes IP3 = increased calcium = activates calmodulin (protein that binds with Ca2+) = this can activate kinases = phosphorylation of protein
summary - peptide hormone receptors
bind to a cell surface receptor
linked to a Tyrosine K or G protein coupled
G-coupled
1st messenger = hormone (signal through cell surface receptor)
2nd messenger = interactions between intracellular domain + molecules within the cell
steroid hormone receptors
ligands are small lipohilic molecules (bc can get through membrane*)
the receptor is encoded by a single gene
have an ability to bind to DNA (*)
function as transcription factors
interestingly many receptors have been identified with no known ligand (orphan receeptors)
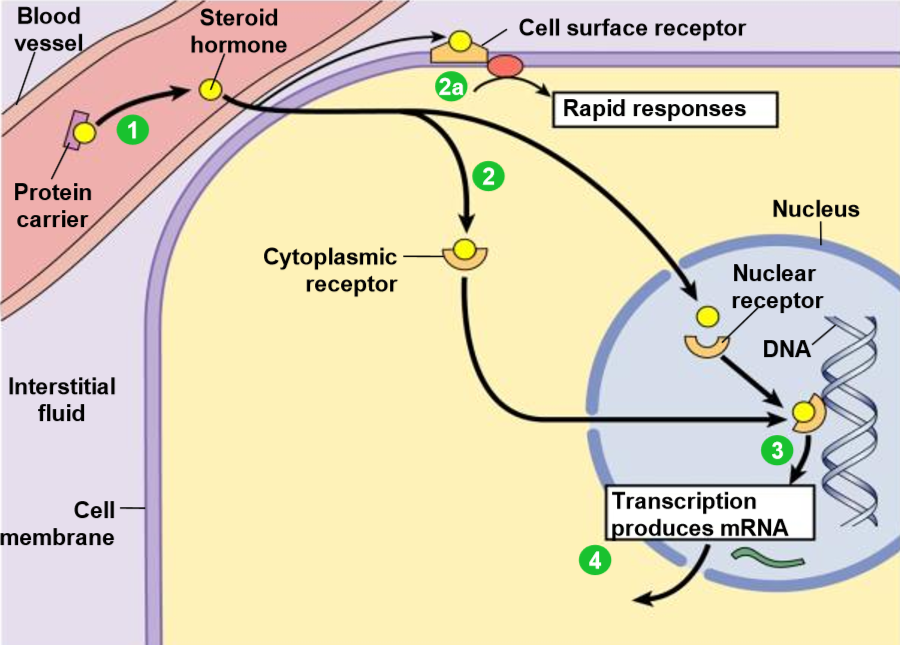
steroid hormone receptors - process
most hydrophobic steroid are bound to plasma protein carriers - only unbound hormones can diffuse into target cell.
hormones jump on/off protein carriers to pass through membrane.
the hydrophobic steroid hormones are able to travel through blood bc they have protein carriers
steroid hormones receptors are typically in the cytoplasm or nucleus.
2a. some steroid hormones also bind to membrane receptors that use 2nd messenger systems = rapid cellular responses.
the receptors-hormone complex binds to DNA + actives/represses one or more genes
activated genes create new mRNA that moves into the cytoplasm
translation produces new proteins for cell processes
homodimers - type I
e.g. glucocorticoid, mineralocorticoid, oestrogen, androgen receptors
ligand binding to type I nuclear receptors in the cytosol results in:
dissociation of heat shock proteins
homo-dimerisation
translocation (i.e. active t) from the cytoplasm into cell nucleus
binding to specific sequences of DNA - known as hormone response elements (HRE’s)
the nuclear receptor/DNA complex then recruits other proteins which transcribe DNA downstream from the HRE into mRNA = protein = change in cell function
heterodimers - type II
e.g. VDR, RAR, TR heterodimerise
type II receptors are retained in the nucleus regardless of ligand binding status
bind as heterodimers to DNA (usually with RXR)
heterodimers = both protein partners are different
in the absence of ligand, type II nuclear receptors form complexes with co-repressor proteins.
ligand bindings to the nuclear receptor causes dissociation of co-repressor + recruitment of co-activator proteins.
if they are already bound to the response element = why is gene not active?
Bc there is co-repressors which stop it working
When we want it to work = they are replaced by co-activators
additional proteins including RNA polymerase are recruited to the NR/DNA complex which translate DNA into messenger RNA.
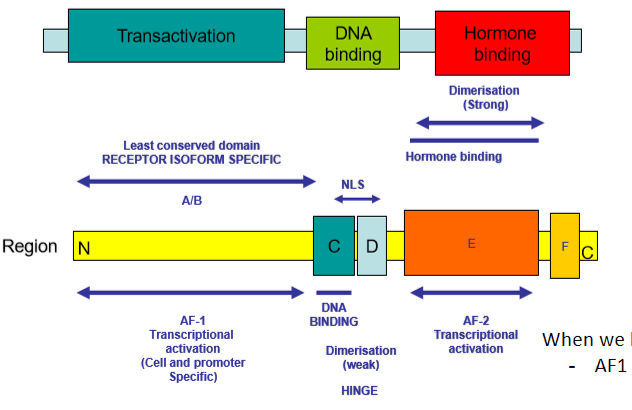
structure of hormone receptor cell
3 overarching domains
Hormone binding
DNA binding
Transactivation - allows protein to be expressed
In hormone binding domain:
There is region AF-2 - transcriptional activation part
In transactivation domain:
There is AF-1
When AF-1 and AF-2 work together to upregulate gene expression
basc: When we bind hormone to hormone binding domain
AF1 + AF2 work together to increase the expression of that gene
nuclear receptors proteins
nuclear receptor proteins are transcription factors
hormone binds to receptor = binds to DNA to control gene expression
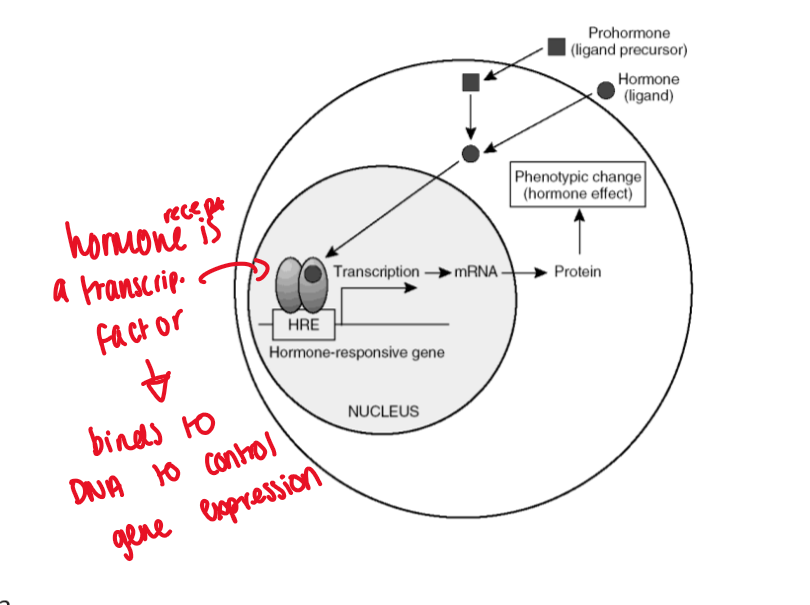
hormones change the pattern of gene expression
promoter region - region of DNA where RNA polymerase attaches and initiates transcription
Gene - area of DNA which codes mRNA
ligated steroid receptor dimers bind to unique regions in the promoter region of genes
Zinc finger
1st zinc finger domain binds DNA
zinc finger = DNA binding protein
holds zinc there = enables it to dock to DNA
2nd zinc finger - involved in dimerisation
hormone response elements (HRE)
short 6 base-pair sequences that allow specificity to occur
can be palindromic, direct repeats, inverted repeated etc.
P-box
this is how a receptor recognises a specific HRE
think of it as a box that contains things
contains:
zinc fingers
elements that allow it to bind to DNA
HRE recognition sequences - will be able to recognise that specific area of DNA
summary - nuclear receptors
cytoplasmic or nuclear
ligand-dependent transcription factors
NH2 terminal constitutive transactivation domain
centrally conserved DNA binding domain - zinc fingers
bind DNA via P-box
COOH terminal ligan binding domain and AF2
recognise HRE