Enthalpy/Specific heat
0.0(0)
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
1
New cards
write the formula for solving enthalpy
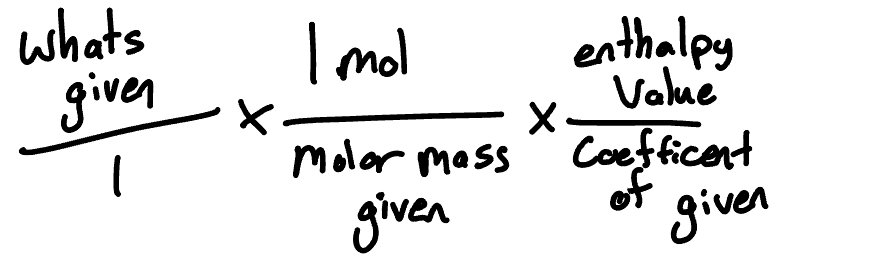
2
New cards
What is specific heat
the amount of hear required to raise the temperature of one grams of a substance by one degree Celsius
3
New cards
In specific heat if a number is higher what does that mean
it takes a longer time to heat up and cool down
4
New cards
In specific heat if a number is lower what does that mean
it heats up and cools down faster
5
New cards
What is the formula for specific heat
Q=MC(triangle)T
6
New cards
What do all the variables stand for in the specific heat equation
Q= head energy(joules)
M= Mass(g)
C= specific heat(J/g degrees Celsius)
(Triangle) T= change in temperature(Celsius)