Chem test 4 things to remember
1/28
Earn XP
Description and Tags
Things to remember para the test
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
29 Terms
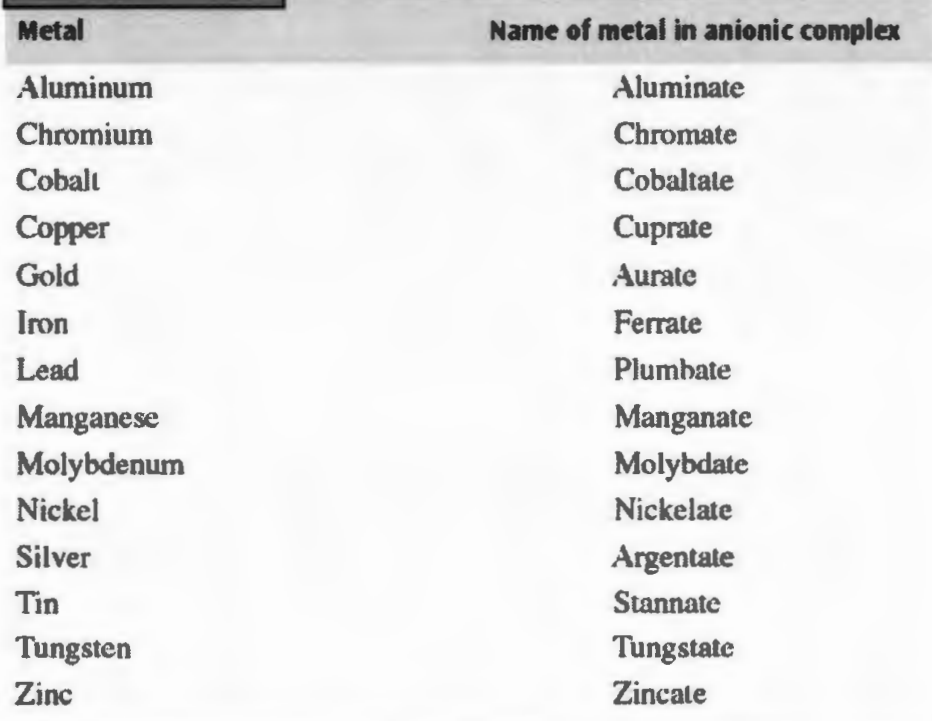
Names of metals in anionic complex
Copper: cuprate
Lead: Plumbate
Iron: ferrate
Silver: Argentate
Tin: stannate
Bidentate ligands
en
Oxalate ion (C2O4 2-)
Polydentate ligand
Ethylenediaminetetraacetate
Has 6 donor atoms
Optical Isomerism
Octahedral
Metal ion has 3 bidentate ligands OR
2 bidentate and 2 monodentate ligands
Example of optical isomerism with 3 bidentate ligands
[Co(en)3]3+
![<p>[Co(en)3]3+</p>](https://knowt-user-attachments.s3.amazonaws.com/dd374abd-179b-4cd0-bf26-578e6464ce9e.png)
Example of optical isomerism with 2 bidentate and 2 monodentate
cis-[Co(en)2Cl2]+
![<p>cis-[Co(en)2Cl2]+</p>](https://knowt-user-attachments.s3.amazonaws.com/18875e45-5c88-4cfc-b31c-c7e148588311.png)
Geometric (Cis-trans) isomer
come in pairs
-Main element with 2 pairs of ligands on side
-90 degrees on cis
-180 degrees on trans
Ex: cis-[Pt(NH3)2Cl2]
![<p>come in pairs</p><p>-Main element with 2 pairs of ligands on side</p><p>-90 degrees on cis</p><p>-180 degrees on trans </p><p>Ex: cis-[Pt(NH3)2Cl2]</p>](https://knowt-user-attachments.s3.amazonaws.com/5e201a45-0aed-430e-a630-e5e919c302f7.png)
Coordination Isomer
ligands can switch spots with outside counter ion
Linkage isomer
Can attach ligand to metal through different isomers
Ex:

Strong ligand
Large delta
Low spin complex
Weak ligand
small delta
high spin complex
Diamagnetic
All electrons are paired
Paramagnetic
Has unpaired electrons
How to know if something will be colorless
has d0 or d10
What absorbs the longest wavelength?
Smallest delta (weakest field ligand)
What absorbs the shortest wavelength?
Largest delta (strongest field ligand)
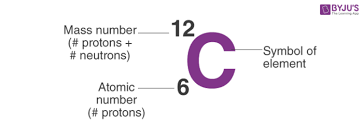
What is mass # and atomic #
Mass # top
Atomic # bottom
Nuclear Fusion
Go from smaller to bigger nuclei
Nuclear Fission
Bigger to smaller nuclei
What number is the minimum to be radioactive?
Z > 83
Proton
1/1 P and 1/1 H
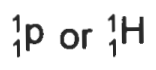
Positron
0/+1 e or 0/+1 B
Neutron
1/0 n
Alpha particle (or He nucleus)
4/2 He or 4/2 alpha
electron (or B particle)
0/-1 e or 0/-1 B
What to do if have too many neutrons? (above belt of stability)
Beta particle emission
What to do if you have too few neutrons? (below belt of stability)
positron emission
or electron capture
Abbreviated notation for bombardment reaction
Reactant (bombarded particle, emitted particle) product
What two isotopes are always radioactive?
Technetium (z=43) Tc
Promethium (z=61) Pm