NS 1150 Prelim 3
1/148
Earn XP
Description and Tags
L14-L20, A2, A3
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
149 Terms
What are Vitamins
Organic molecules found in both plants & animal-derived foods required in small amounts to maintain body's basic functions
13 known Vitamins
Serve as Coenzymes(not all)
What are the essential vitamins(micronutrients) for survival
Vitamin A - Vision
Vitamin C - Scurvy
Vitamin D - Bone Health
Vitamin K - Coagulation
What Vitamins can be Obtained without Food
Vitamin D - UV rays from sun to skin
Vitamin K - activity of intestinal bacteria
Vitamins Synthesized from Diet or Dietary Provitamins
Beta-carotene (provitamin A), naturally found in food, can be converted into active form of vitamin A called retinol
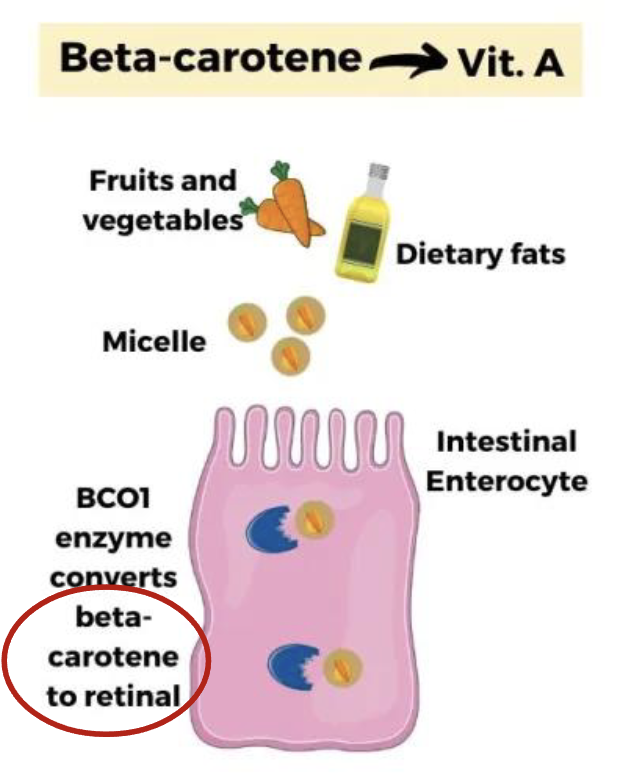
What are Provitamins
Vitamin precursors that can be converted to the active form of a Vitamin
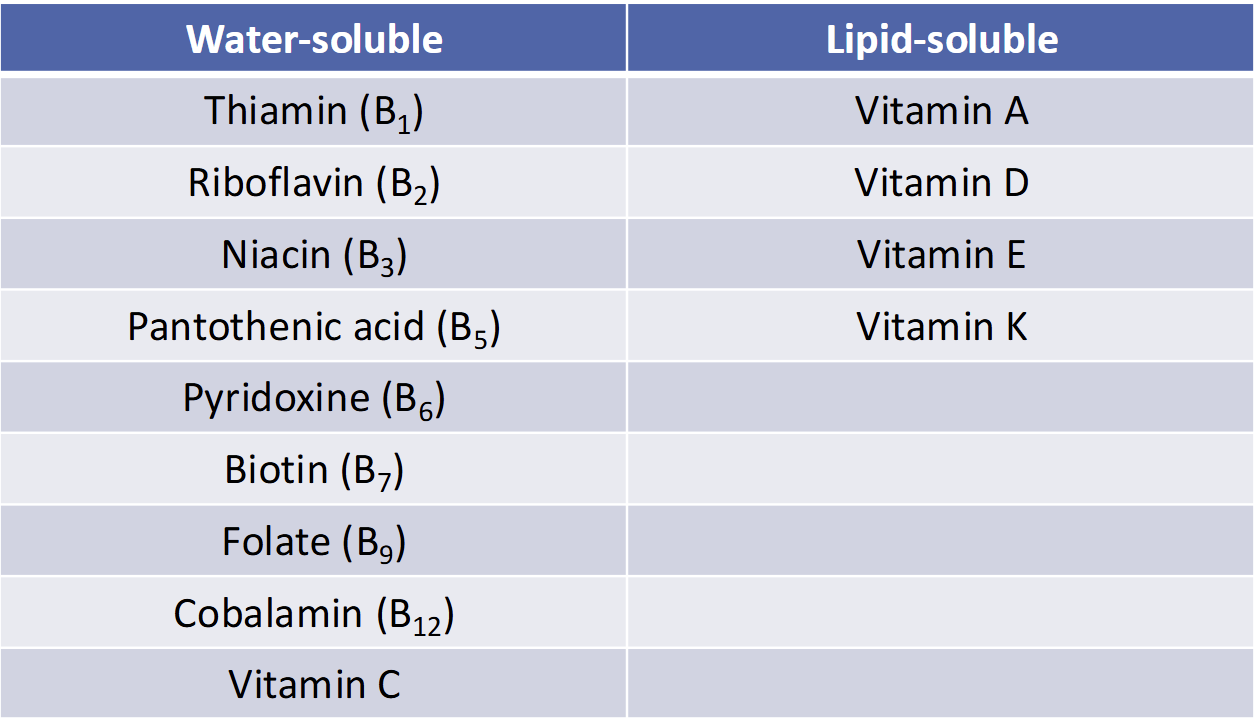
Classification of (13) Vitamins
Water Soluble
All Vitamin B
Vitamin C
Lipid Soluble
Vitamins A, D, E, K
What are 2 conditionally essential vitamins-like compounds that are water-soluble
Choline & Carnitine (endogenously synthesized & Doesn’t act as coenzymes)
Water Soluble vs Lipid Soluble
(Supplies, Body Stores, Toxicity, Absorption, Stability in Food Processing)
Water Soluble = Short term supply available, little storage, low risk of toxicity, easily absorbed into the blood, less stable in food (breaks down easily)
Fat Soluble = Long term supply available, stored in adipose, higher risk of toxicity, absorbed into lymph like lipids (requires protein carriers to travel in blood), more stable in food
What is Bioavailability & What Influences It(5)
Proportion of a nutrient in food that is absorbed & used by the body
Influenced by:
Substances in food
Nutritional Status
Age (producing less HCl as you age prevents you from accessing and separating B12 from the food matrix)
Medication (lots of antacids lead to decreased HCl)
illness (kidney disease alters needs for nutrients)
How are vitamins destroyed by Heat
Heating at extreme temperature for long period of time desotry most of the water soluble vitamins
Recommendation: don’t overcook fruits & vegetabls
How are vitamins destroyed by Light
Light destroys several of B Vitamins like milk exposed to light leads to destruction of riboflavin
Recommendation: don’t put milk in glass
How are vitamins destroyed in excess water
Water soluble vitamins can move out of foods when they are placed in water
Recommendation: Don’t soak food or cooked veggies
How are vitamins destroyed by alkaline conditions
High pH(low acidity) can desotry thiamin & vitamin c
Recommendation: avoid adding baking soda to veggies to maintain their colors
How does oxidation destroy vitamins
Vitamin C & many B vitamins are destroyed by exposure to air(oxygen)
Recommendation: Store vitamin-rich food in air-tight containers
What are Coenzymes
Organic Molecules that attach to enzyme & activates/increase enzyme ability to catalyze metabolic reactions
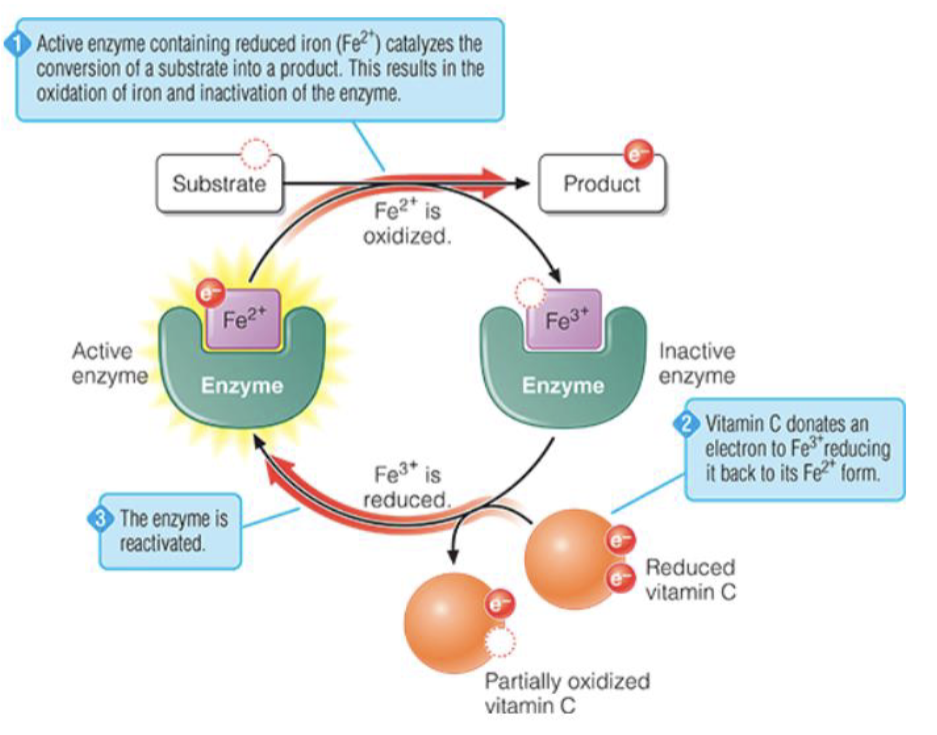
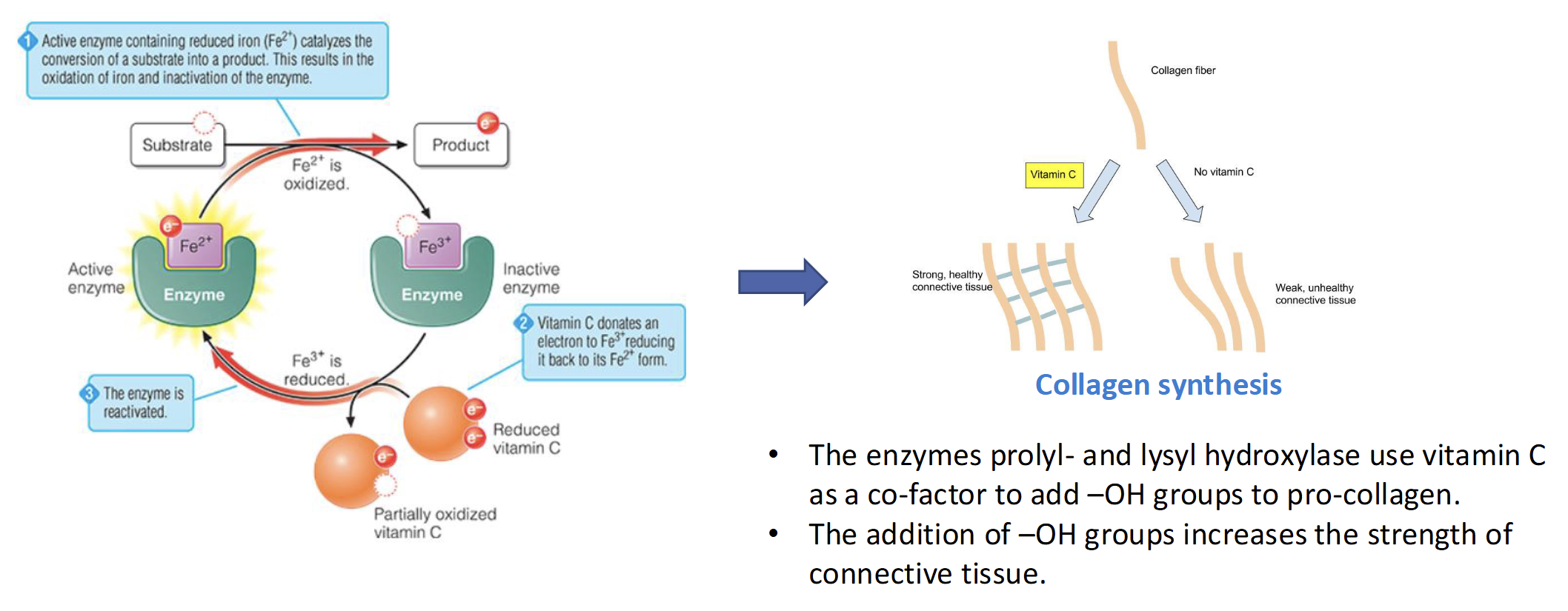
What Vitamin is required for Collagen Synthesis
Vitamin C
Enzyme prolyl- & lysyl hydroxylase use Vit C as a co-factor to add -OH groups to pro-collagen which increases the strength & connective tissue
What is the most common cause of vitamin supplementation
Vitamin Toxicity
Very Rare from Dietary Vitamins
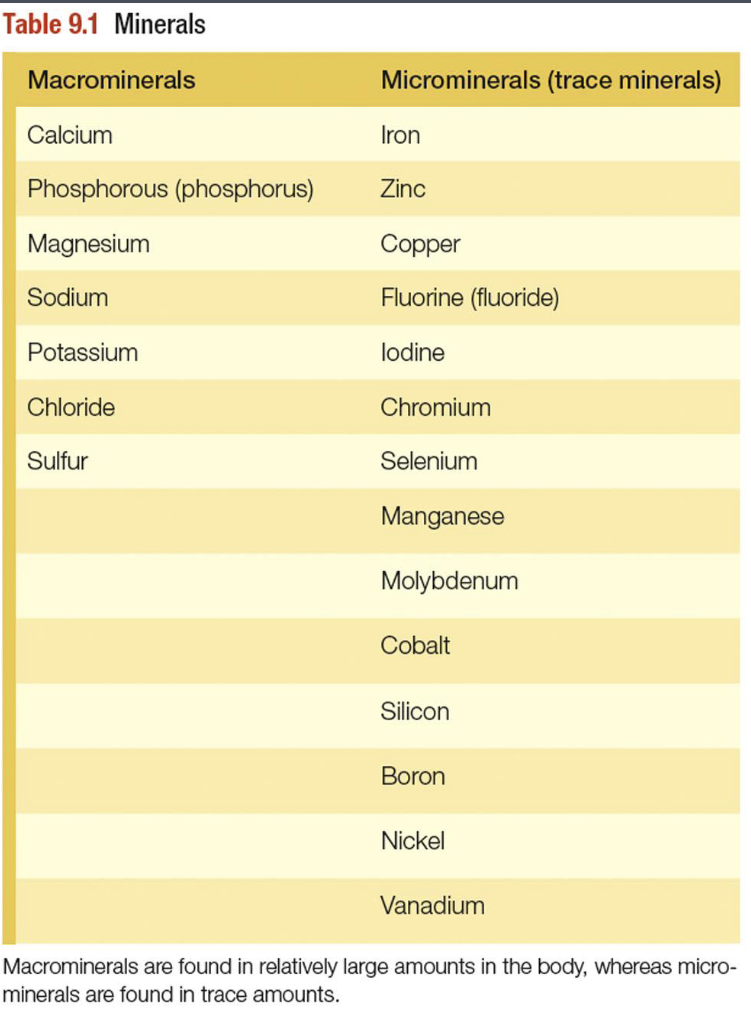
What are minerals
Inorganic molecules, other than water, that are essential for human survival
essential nutrient because body cannot make (animal based have more bioabailability than plant)
Cofactors
Cannot be created or destroyed(not by cooking or digestion)
How are minerals lost
Sweat
Sodium, Chloride, Potassium, Calcium, Iron, & magnesium

Classification of minerals
Macrominerals - Needed in amounts >100mg/day
Microminerals(trace minerals) - needed in amounts less than or equal to 100 mg/day
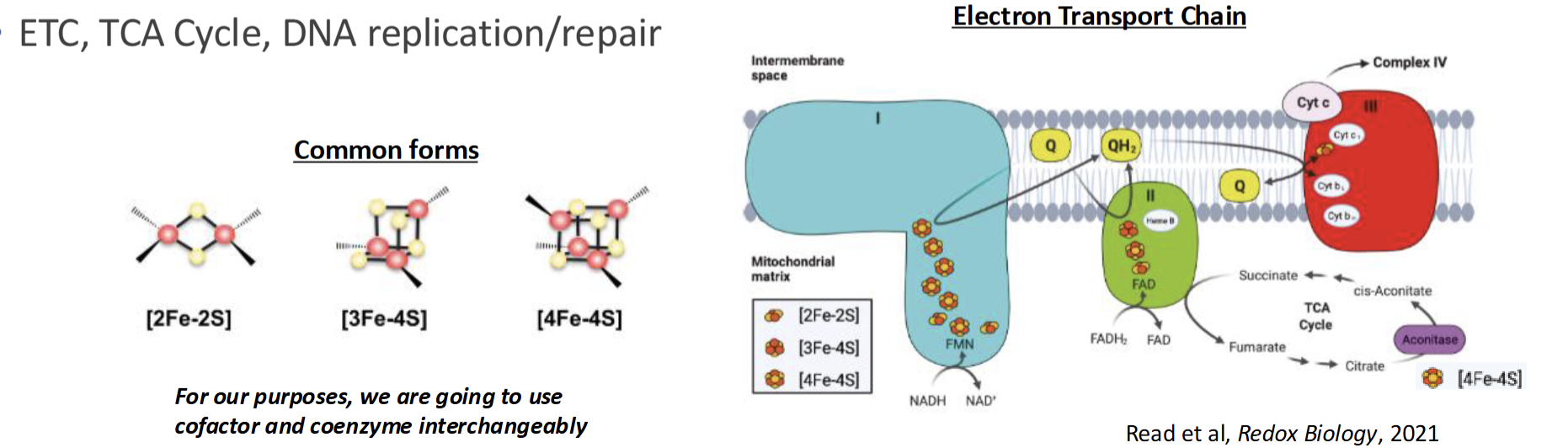
What are some example mineral cofactors
Mg acts as cofactor for DNA synthesis
Iron & Sulfer combine into Fe-S clusters and is most common cofactor observed: ETC, TCA, DNA repair
Mineral Toxicity
Rare due to tight regulation of absorption, excretion, & storage
Body stores small amounts of certain minerals
Exception: genetic disorder or overconsumption of mineral-containing supplement
What is Micronutrient Fortification
adding nutrients to foods that were either not present or present in small amounts before processing
Reason to Fortify Foods (4)
Correct Dietary insufficiency in population
Iodine salt for goiters & folic acid for NTDs
Restore nutrients to original levels after processing
Thiamin is added to white rice which is lost with milling
Provide balance
Breakfast cereals to make them more nutrient dense
Prevent nutritional inferiority in food that replace traditional food
Adding calcium & vitamin D to milk products like soy or almond
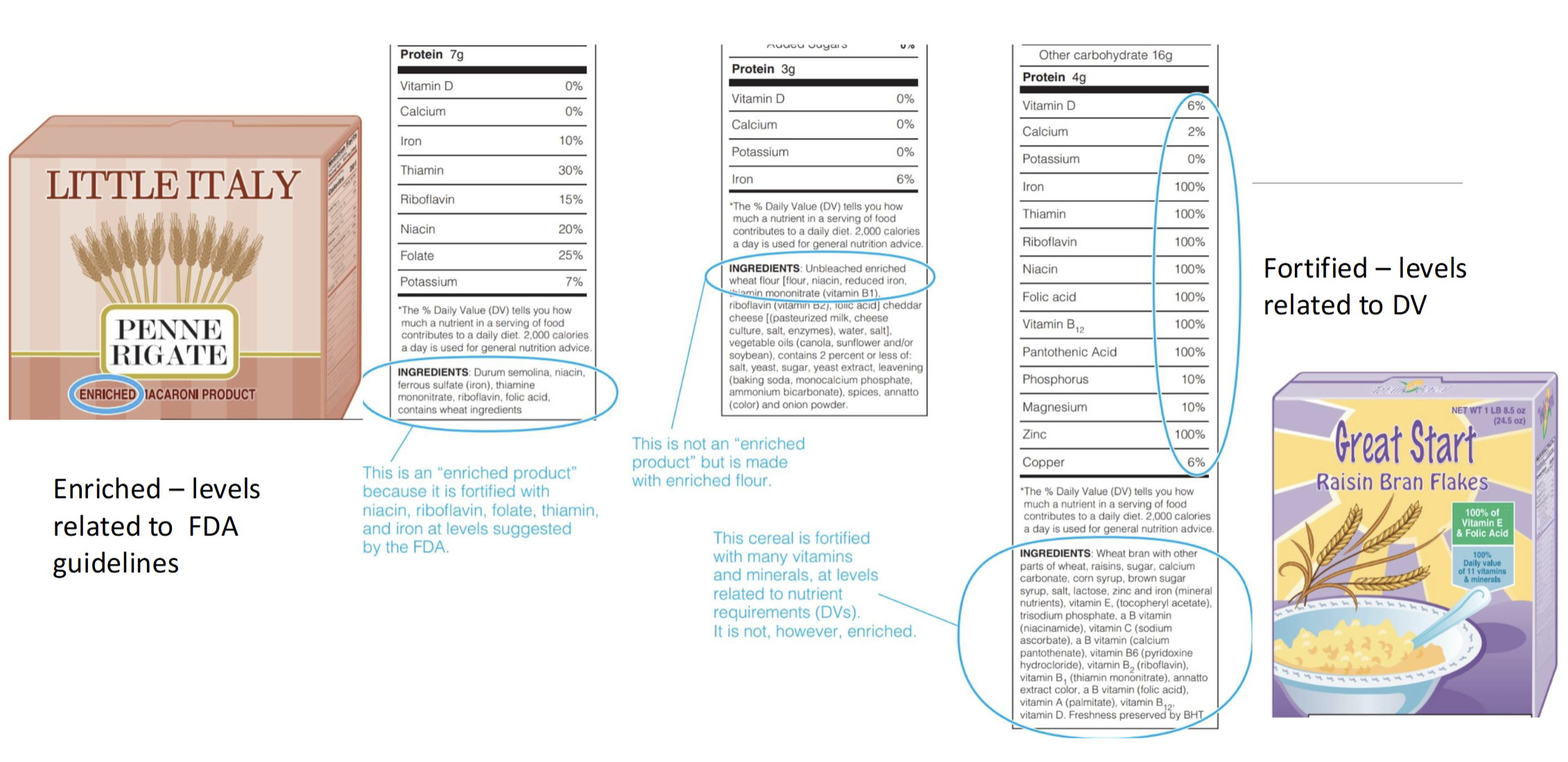
What is Micronutrient Enrichment
A type of fortification involving addition of specified amounts of sets of certain nutrients to a limited list of foods
Set by US FDA
Only select foods are labeled as enriched(whole grains, grain products & flour)
Must include: 4 B vitamins(thiamin, niacin, riboflavin, & folic acid), Mineral Iron, addition of calcium optional
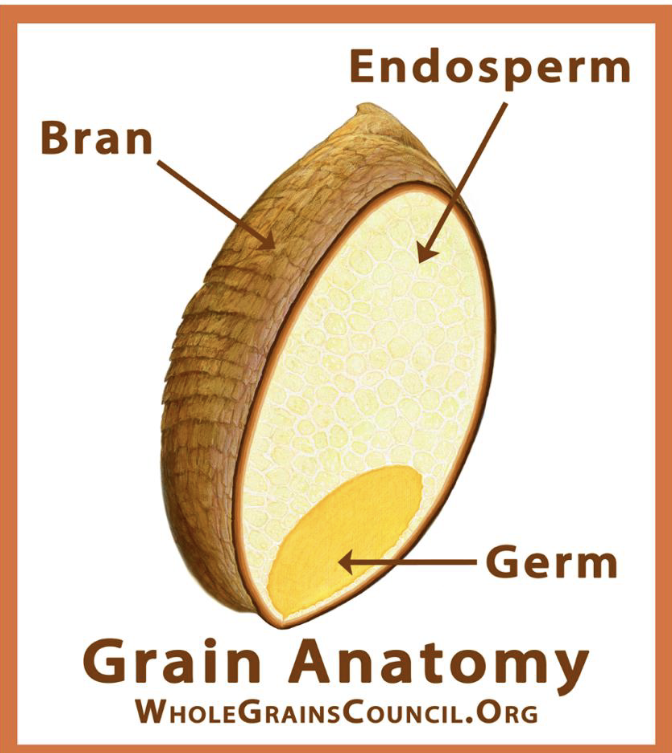
Anatomy of a Grain Kernel
Bran - rich in antioxidants, B vitamin, fiber
Endosperm - essential nutrients to plants; rich in starchy CHO, small amount of proteins, vitamin, & minerals
Germ - Core of seed, rich in B vitamin, protein, mineral, PUFA
What are Refined Grain
Missing one or more of their anatomical parts(bran, germ, endosperm) which impairs nutritional value

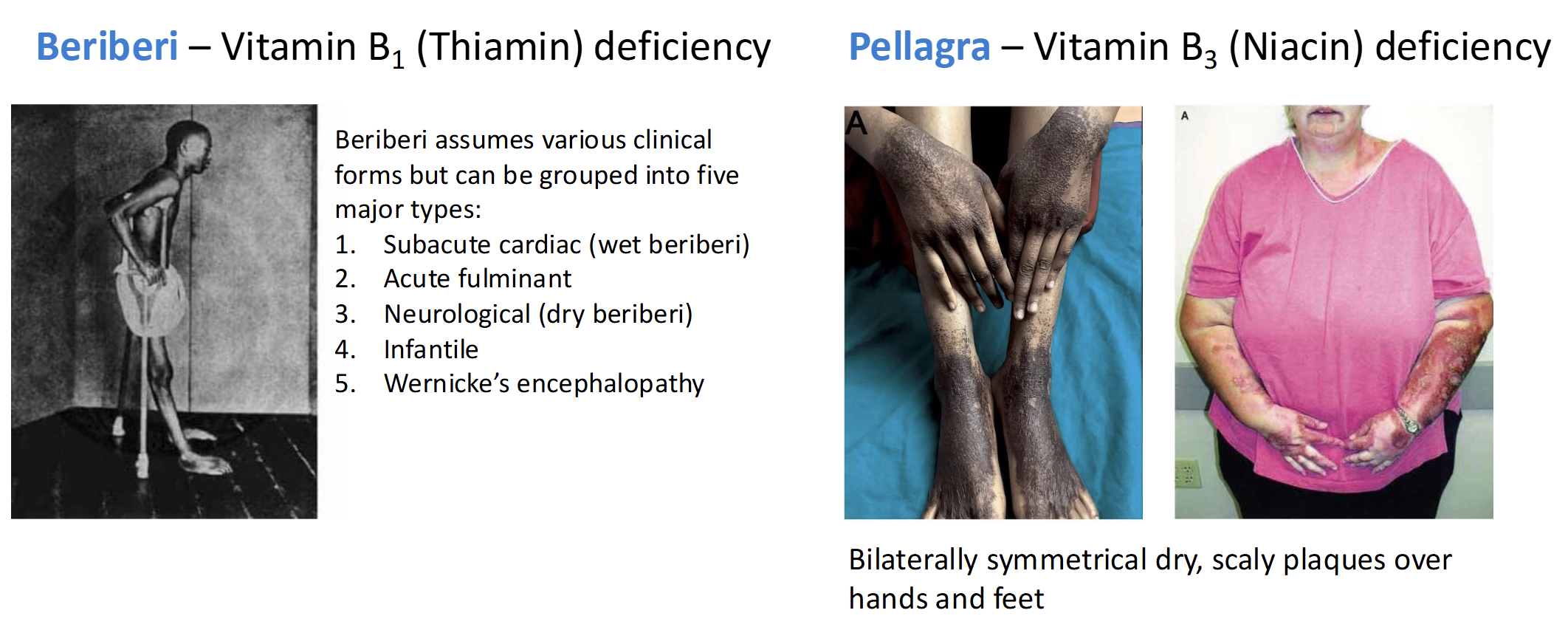
Nutrient Deficiencies of Refined Grain Consumption
Beriberi - Vitamin B1(thiamin) deficient
Pellagra - Vitamin B3 (Niacin) deficient
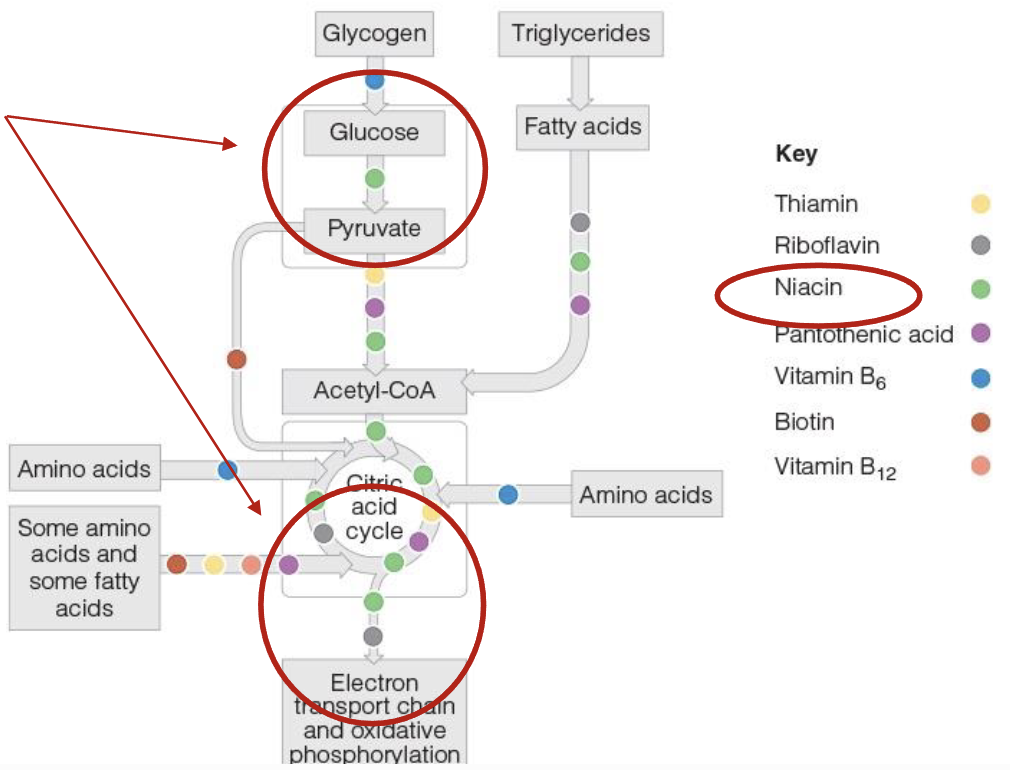
Role of Thiamin(B1), Riboflavin(B2), & Niacian(B3) in energy production
Act as coenzymes and assist enzyme in the release of energy from CHO, Lipids, & Proteins
without these coenzymes, their respective enzyme cannot function
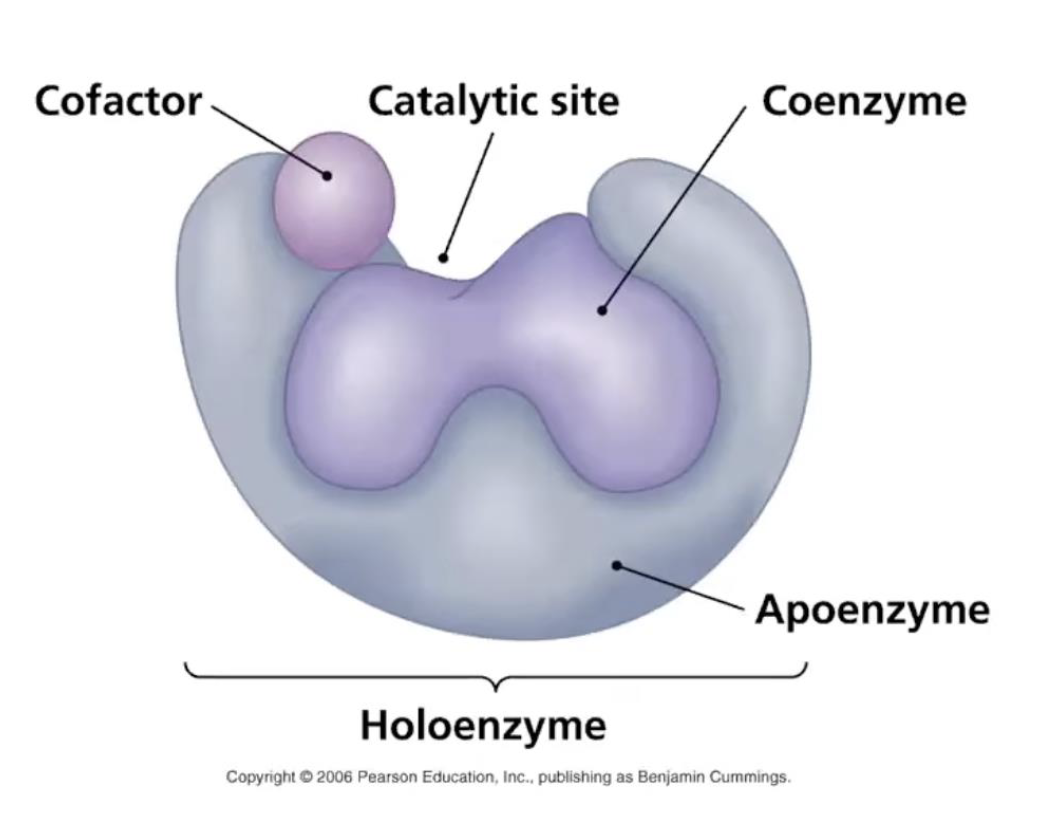
Enzymes- Apoenzyme, Catalytic site, Cofactor, Coenzyme, Holo-enzyme
Apoenzyme - protein part of the enzyme
Catalytic Site - region where enzyme interact w/substrate
Cofactor - Inorganic ion (iron, copper, zinc)
Coenzyme - nonprotein, organic molecule bound or loosely associated with the enzyme (biotin, NAD, FAD)
Holo-enzyme - active form of the enzyme (comprised of the apoenzyme and the coenzyme and/or cofactor
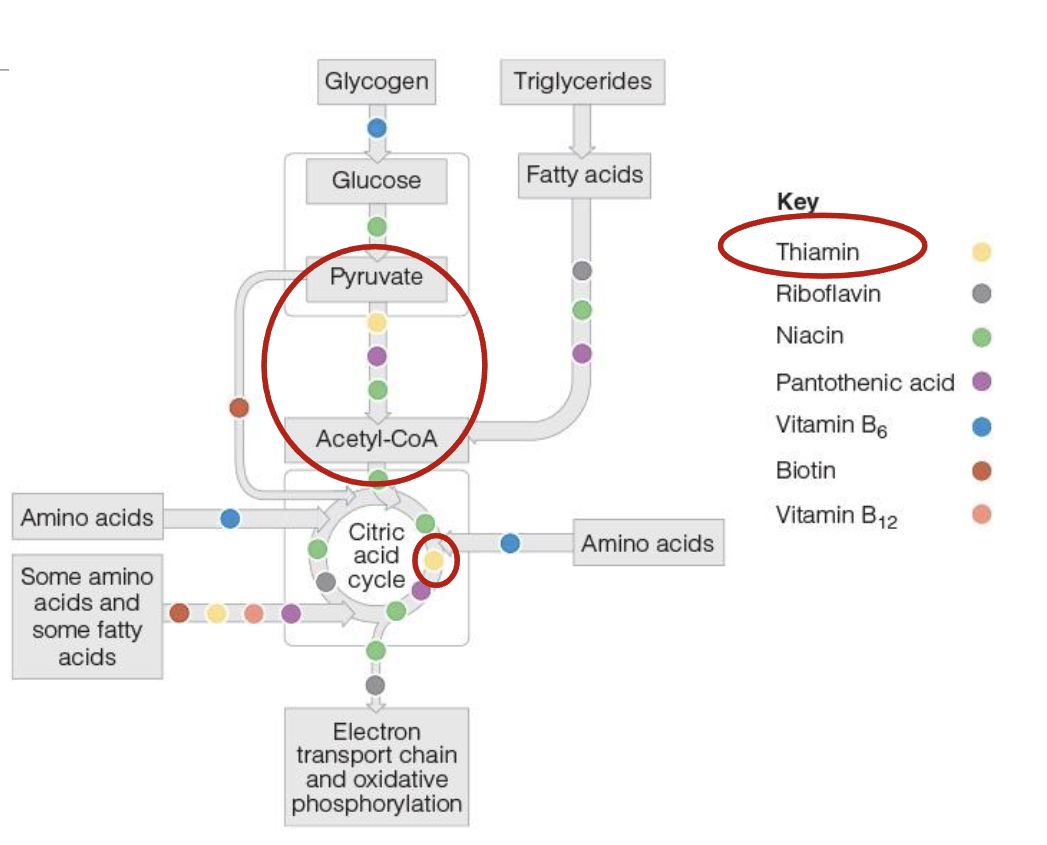
Thiamin(B1) role in energy production
2 phosphate groups are added to thiamin to form coenzyme thiamin diphosphate(TDP), which is essential for energy metabolism
conversion of pyruvate into acetyl-CoA, allowing CHO metabolism to proceed into TCA
In TCA, TDP aids in conversion of 5C compound into a 4C compound
Where else is Thiamine found in the body
Membranes of nerve cells, where it aids in muscle activity
Thiamin (B1) Deficiency Casues
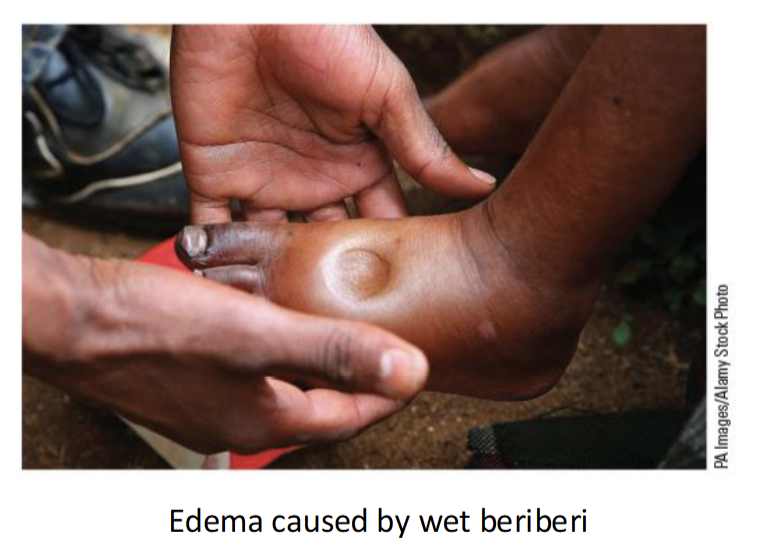
Beriberi(2 types + 1 in baby)
Dry - damage to nervous system; muscle weakness
Wet - damage to cardiovascular system; dilates blood vessels(edema-swollen extremities)
infantile - observed in infants
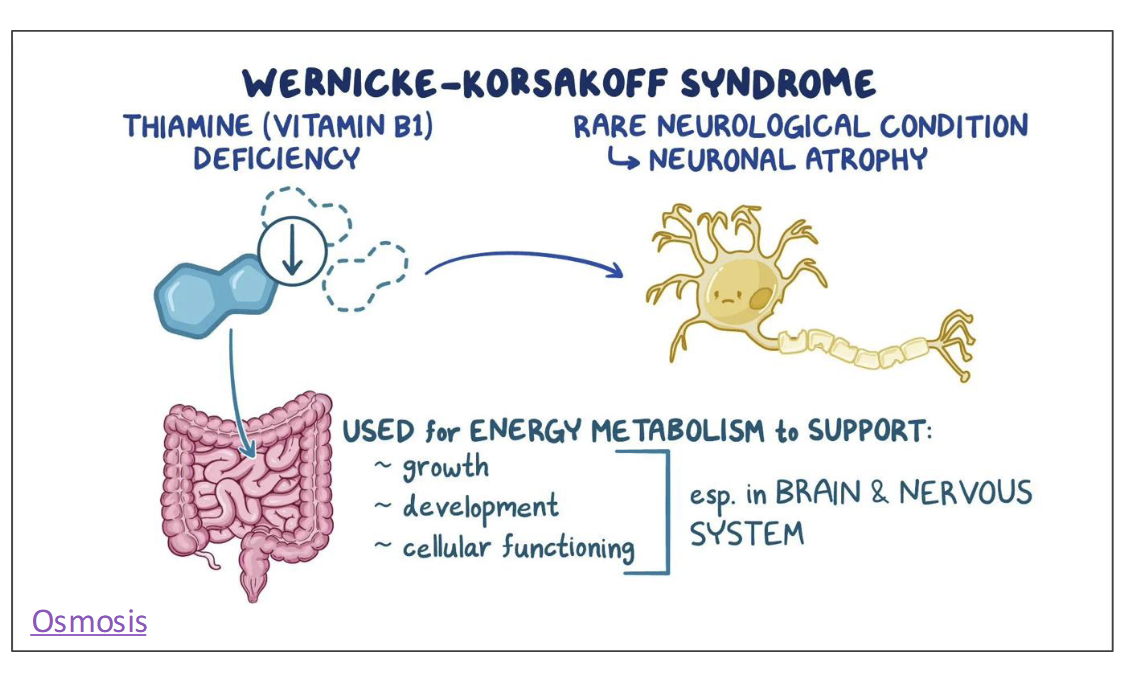
Thiamin(B1) Toxicity
Chronic alcohol consumption can lead to B1 deficiency, a condition called Wenicke-Korsakoff syndrome
Alcohol impairs thiamin absorption & hasten thiamin excretion in the urine
Food Sources of Thiamin
Whole grain, pork, fish
RDA & UL of Thiamin
RDA
Women: 1.1 mg/day
Men: 1.2 mg/day
UL
None has been determined as no adverse effects have been associated with excess thiamin
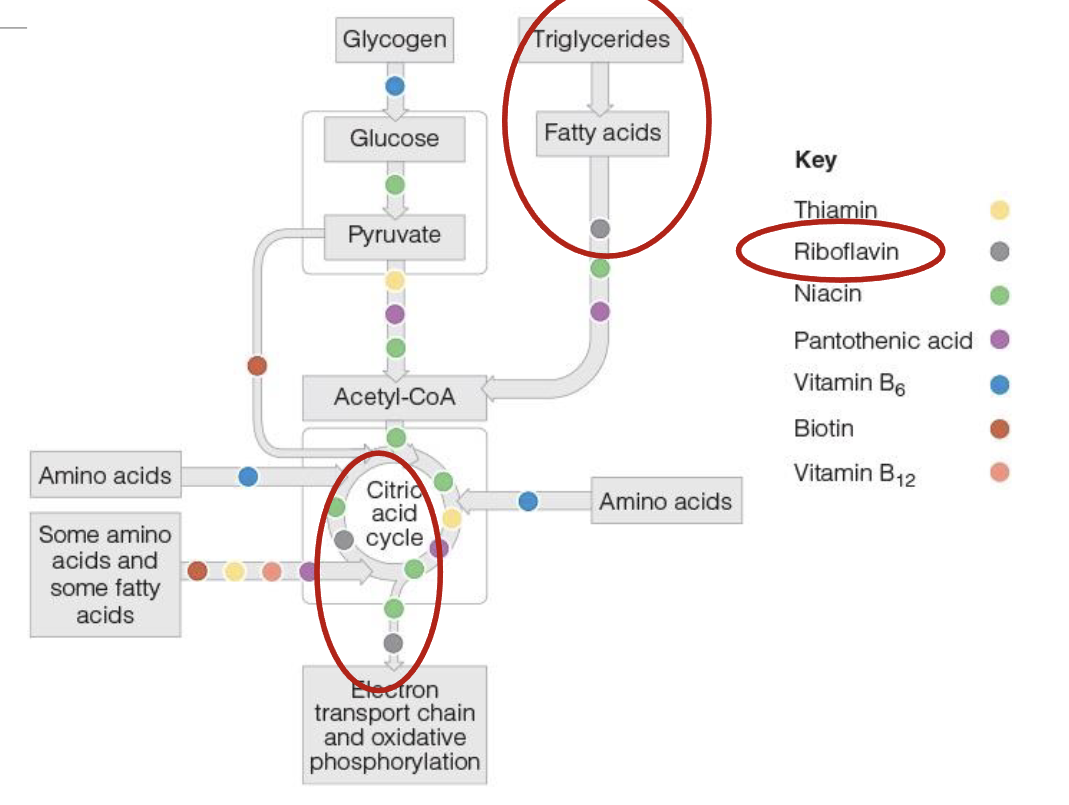
Riboflavin(B2) role in energy production
Coenzyme form of Riboflavin are Flavin Mononucleotide(FMN) & Flavin adenine dinucleotide(FAD)
FAD picks up high energy electron from TCA(FADH2) & delievers to ETC
FADH2 is needed for breakdown or oxidation of fatty acids into acetly CoA
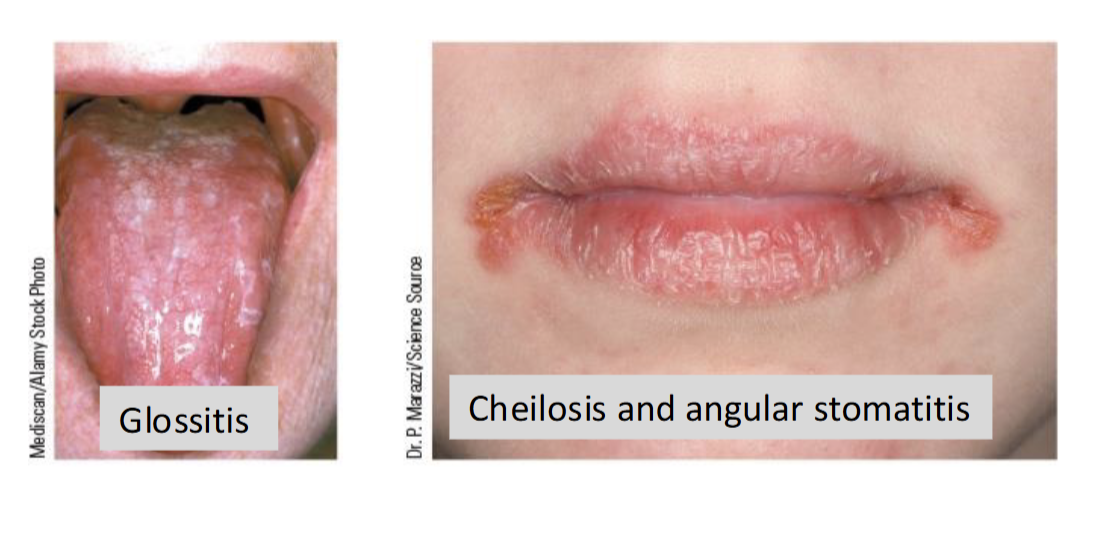
Riboflavin(B2) Deficiency
Rare and typically associated with broader nutrient deficiencies & malnutrition
Causes arbioflavinosis: inflammation of membrane of the mouth, skin, eye, and GI tract
Food Sources of Riboflavin
Meat & Dairy Products
RDA & UL of Riboflavin
RDA
Women: 1.1 mg/day
Men: 1.3 mg/day
UL
No UL has been established as excess riboflavin causes no harm
Niacin(B3) role in energy production
2 coenzyme forms: Nicotinamide adenine dinucleotide(NAD) and its phosphate form NADP
NAD carries hydrogen+electron during metabolic reactions like glycolysis and path from TCA to ETC
NAD protects against neurological degeneration
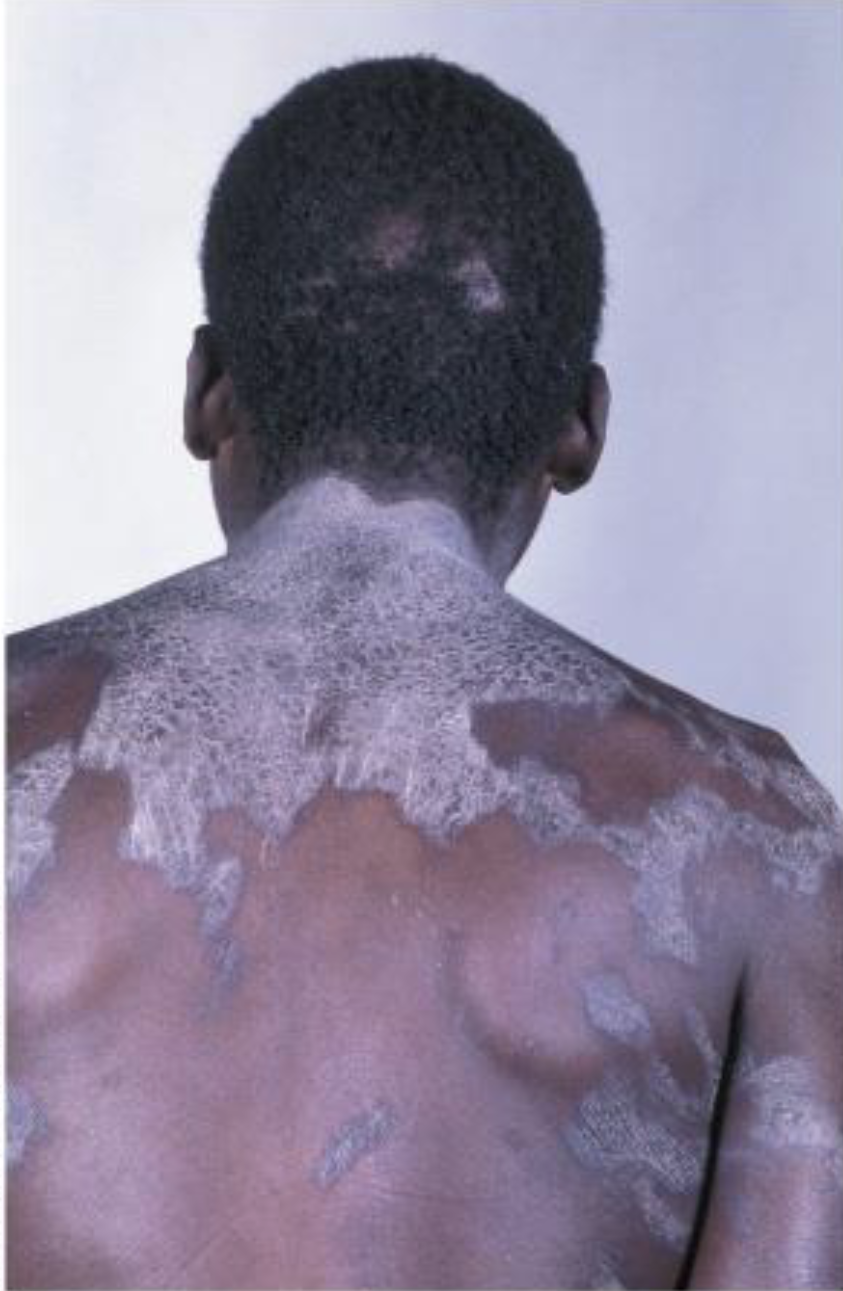
Niacin(B3) Deficiency
Pellagra(Angry skin), which has symptoms of diarrhea, dermatitis, dementia, and death(4Ds)
Humans can synthesize niacin from tryptophan when protein requirements have been met
Niacin toxicity
Large doses of nicotinic acid from supplements is associated with nicotinic flush
doses 3-4x higher than RDA
Dilates capillaries causing tingling sensation/painful
increases plasma glucose & damages liver
Food Sources of Niacin & Conversion
Meat & mushrooms
1 NE(niacin equivalent: including precursor and nician) = 1 mg of niacin =60 mg of trptophan
RDA & UL of Niacin
RDA
Women = 14mg/day
Men = 16 mg/day
UL
No more than 35 mg/day
Micronutrients for Bone Health
Vitamin D, Calcium, Vitamin K
Vitamin D - The “Sunshine Vitamin”
Conditionally essential nutrient
produced in the skin through sunlight exposure or obtained from the diet
Vitamin D - A Prohormone
Prohormones are compounds that the body convert into an active hormone
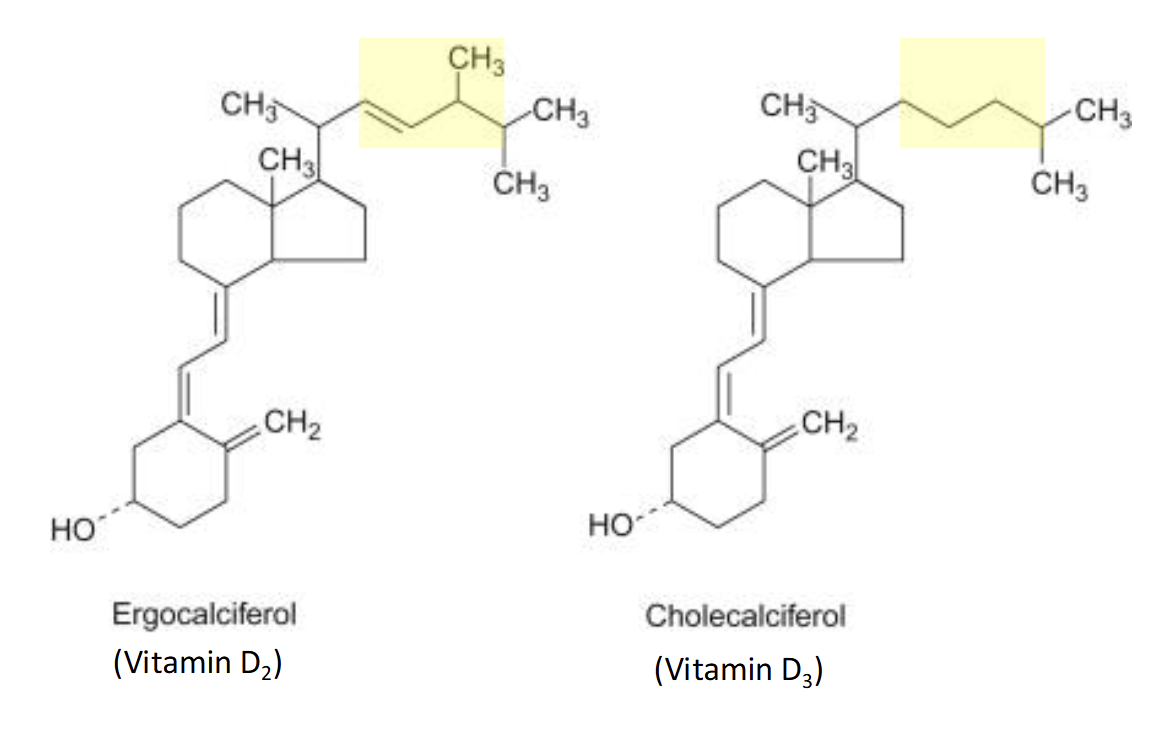
2 Forms of Vitamin D(found as dietary supplements)
Ergocalciferol(D2)
Plant Foods
Cholecalciferol(D3)
Animal Derived foods
Produced in the skin
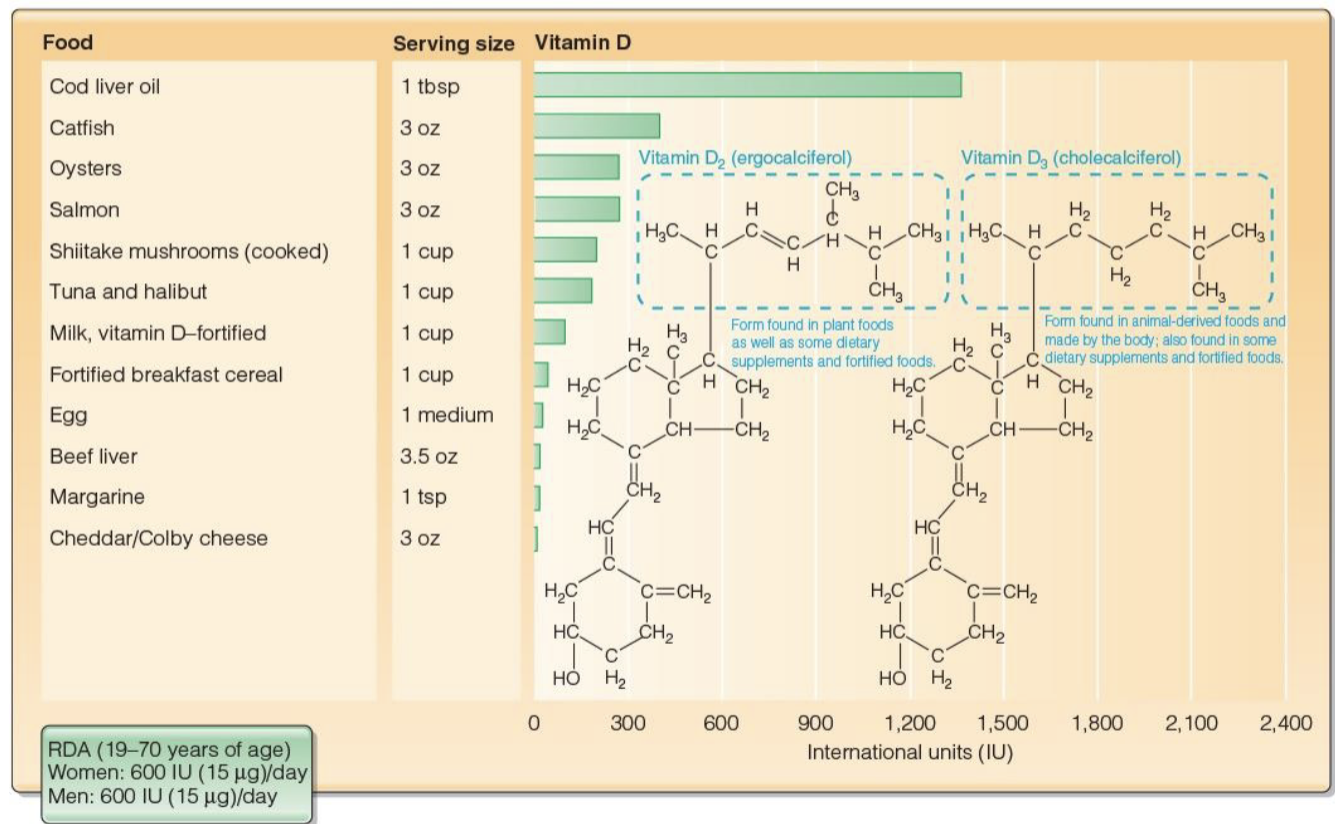
Food Sources Containing Vitamin D(D2 & D3)
Cholecalciferol
Fatty fish(salmon)
Liver Oil
Egg Yolk
Ergocalciferol
Mushrooms exposed to UV light
Both(Fortified Foods)
Dairy Products
Breakfast Cereals
RDA & UL of Vitamin D
RDA
Women - 600 IU=international units (15micrograms/day)
Men - 600 IU (15micrograms/day)
UL
4000 IU or 100 micrograms/day
Overconsumption can lead to harden blood vessels, forming stones(kidney stones)
How is Vitamin D absorbed in the S.I
Same Mechanism as dietary fat
(Lipolysis-Bile-esterification-lymphatic system- chylomicrons)
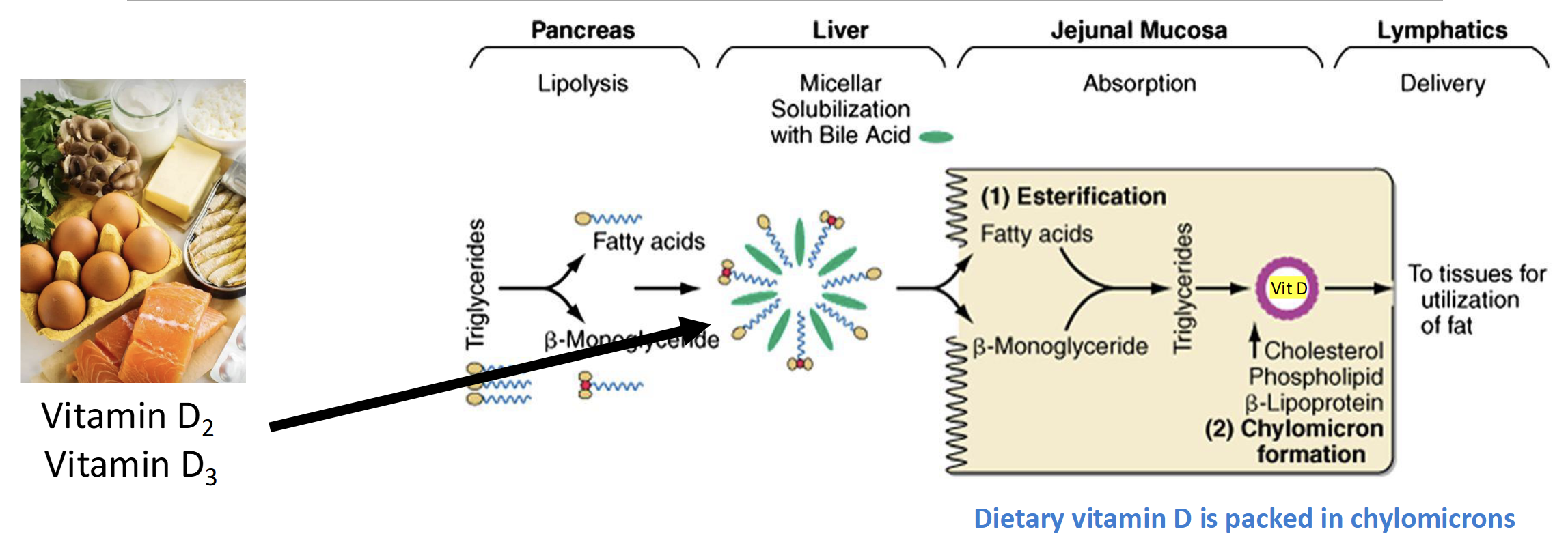
Bioavailability of Dietary Vitamin D
Vitamin D is Stable & Not easily destroyed during food preparation, processing, or storage
Drugs(Barbiturates, anti convulsants,prednisone) desotry Vitamin D
Synthesis of Cholecalciferol (D3)
Exposure of skin to UV light converts 7-dehydrocholesterol converted to previtamin D3(precalciferol) which is then converted to vitamin D(cholecalciferol)
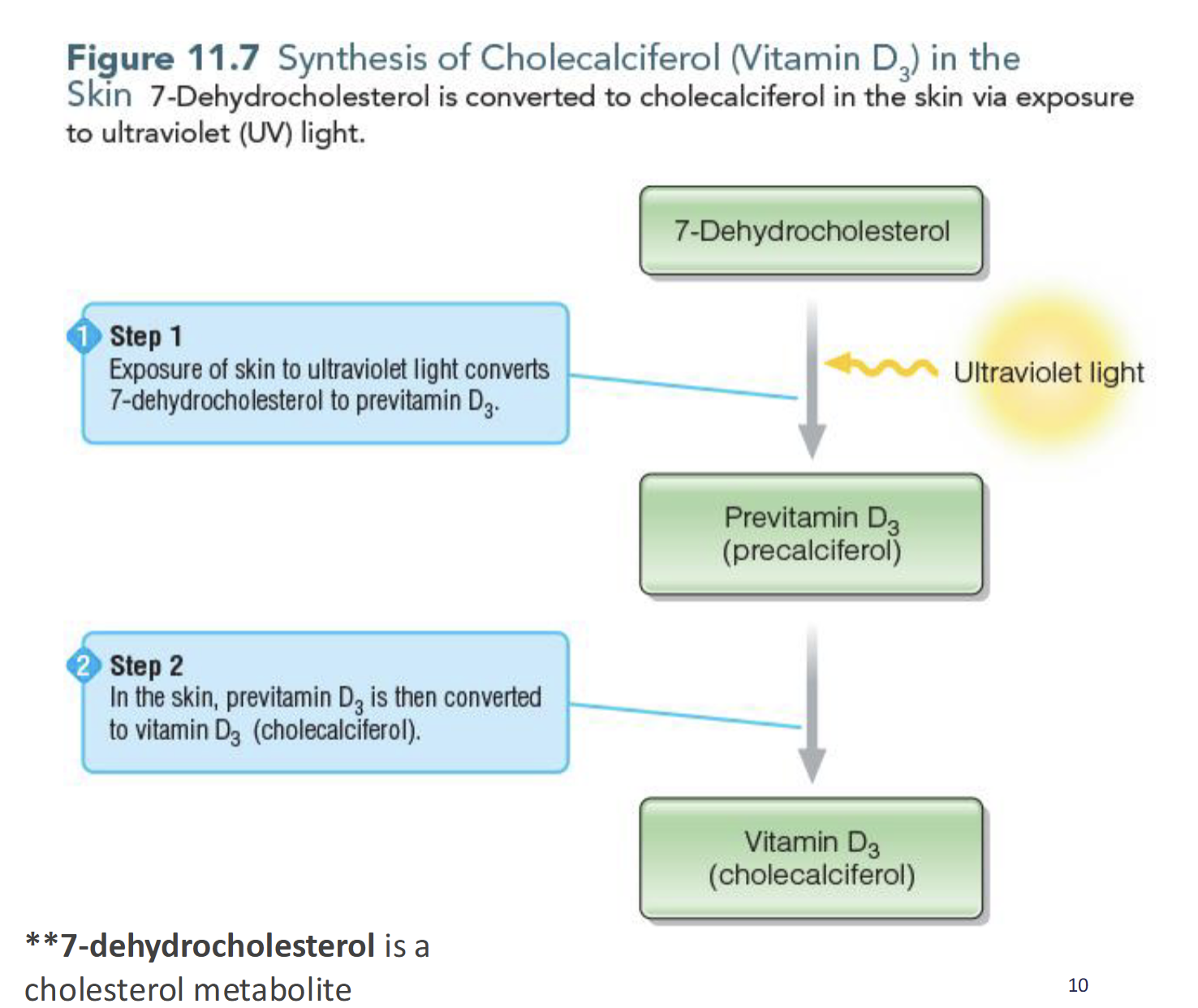
Factors affecting Endogenous Synthesis of Vitamin D
Seasonality, Latitude, time of day
Age, Skin pigmentation(Darker = less Vit D)
Amount of skin exposed, sunscreen
What happens to cholecalciferol(D3) & ergocalciferol(D2)
Liver converts D2 & D3 to 25-hydroxyvitamin D (25(OH)D) which is released into circulation and goes to the kidney
Specific enzyme in kidney converts 25(OH)D to calcitriol (1,25(OH)2D) the active form of vitamin D and a hormone
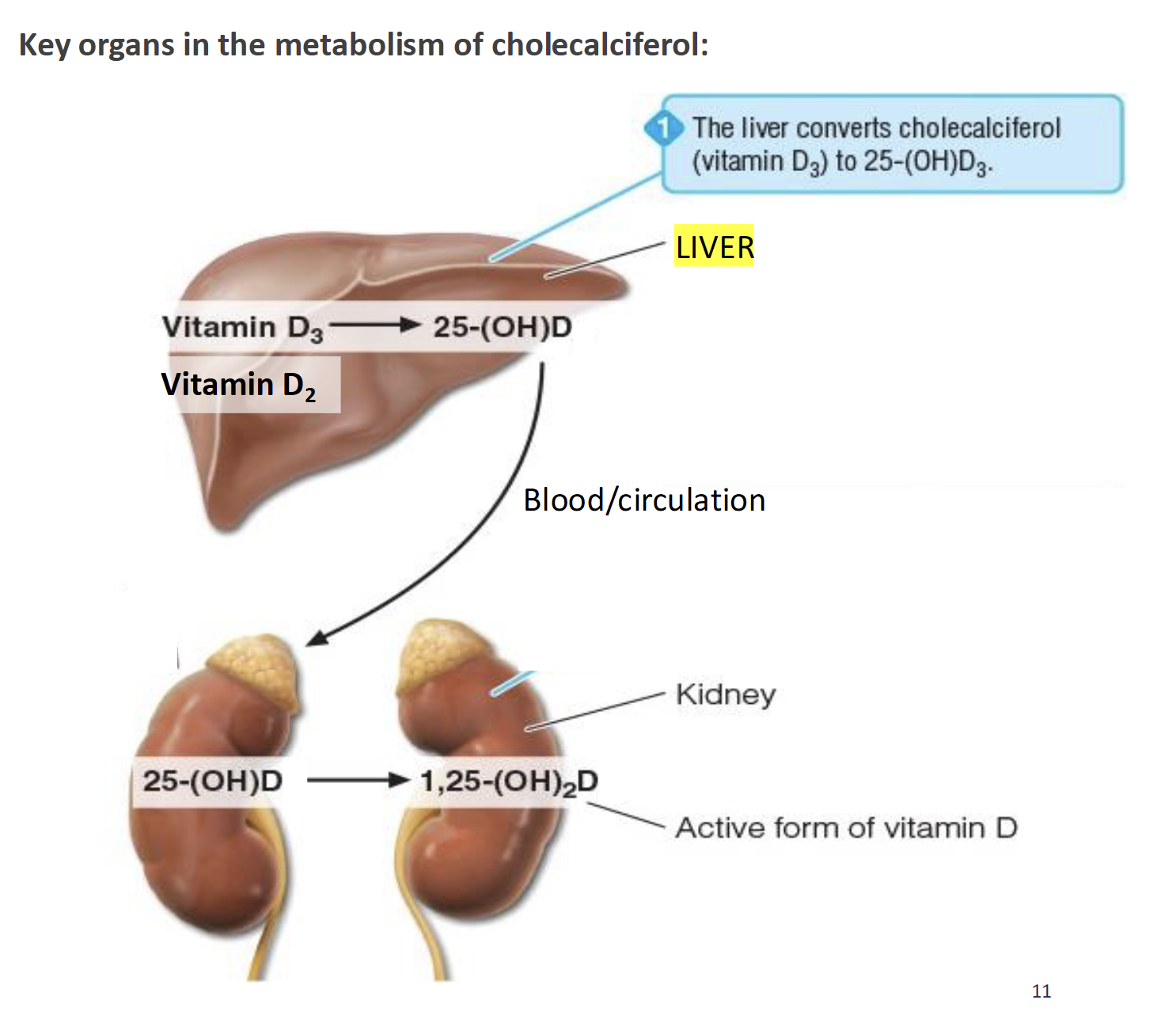
Importance of 1,25(OH)D or calcitriol
Calcium homeostasis
Regulates Gene expression
Regulates cell differentiation
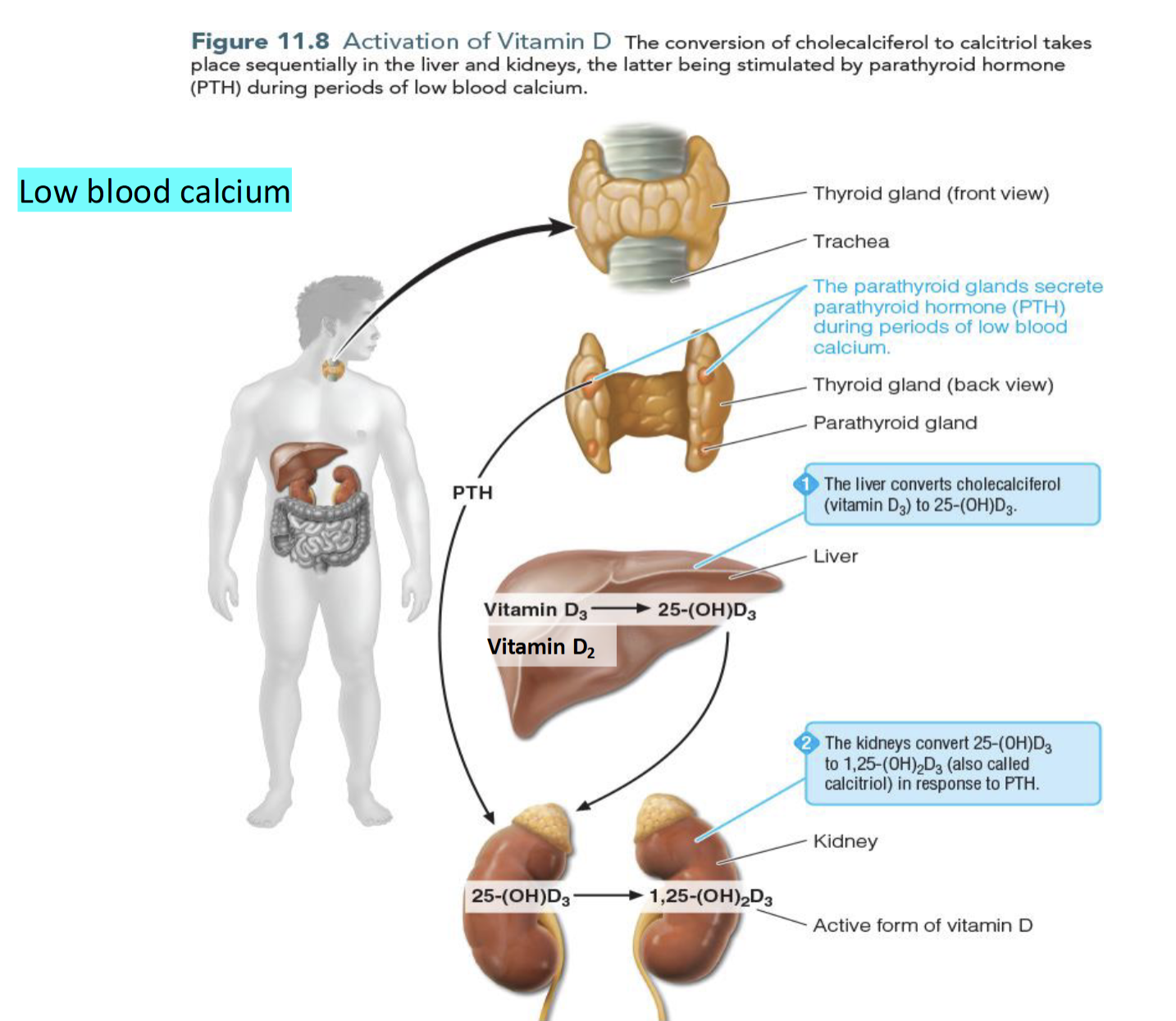
Calcium Homeostasis (low calcium levels)
Parathyroid Gland secretes Parathyroid hormones(PTH) which stimulates the conversion of 25(OH)D to 1,25(OH)2D thereby
increasing dietary calcium absorption
decrease calcium excretion
increase calcium release from bones
Vitamin D deficiency (Relatively Common)
Rickets - inadeqaute bone mineralization in early life(children) leading to softened
Osteomalacia - softening of bones due to inadequate bone mineralization in later years(adults)
Osteoprosis - demineralization of previously healthy bone; weak brittle porous bone
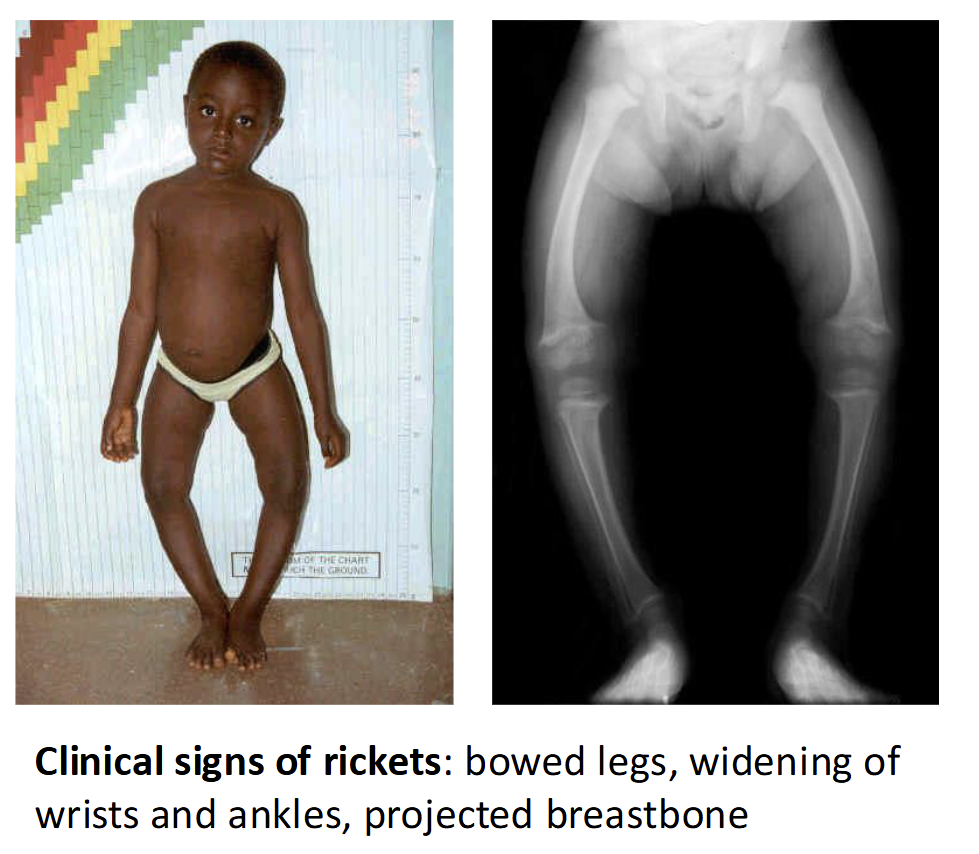
Calcium
Most abundant mineral in the body
99% is in the skeletal system
Considered a nutrient of public health concern(underconsumed)
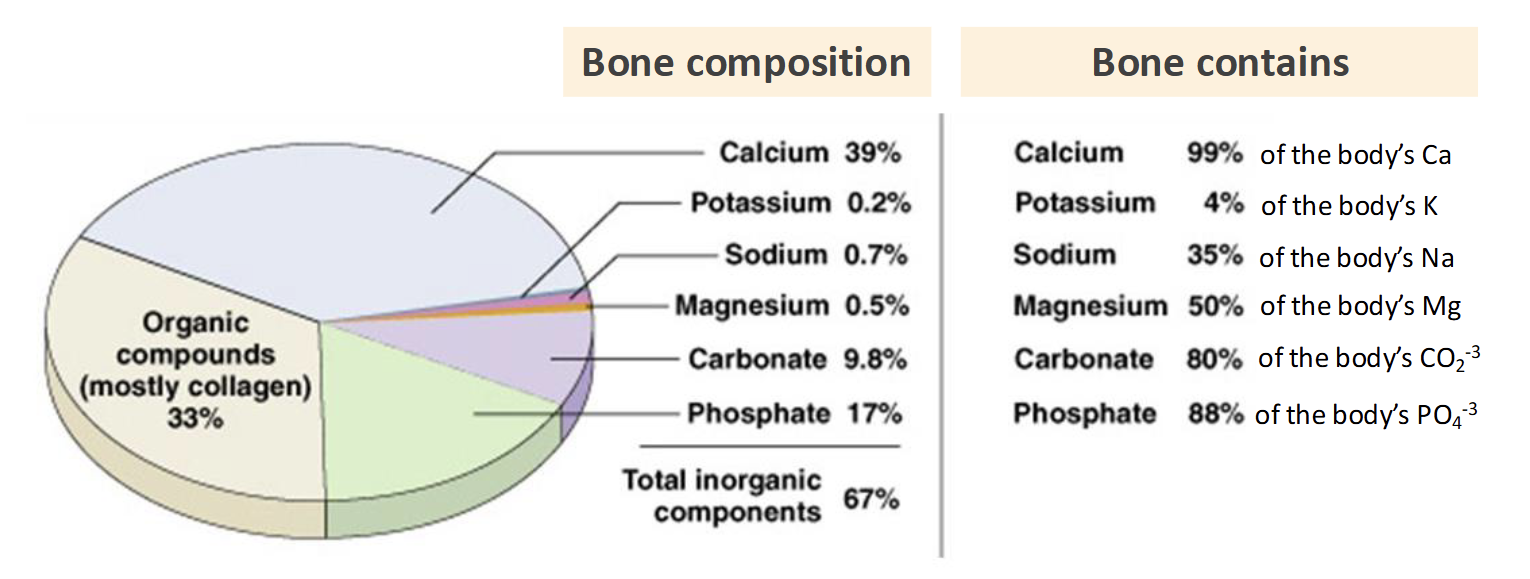
Food Sources of Calcium
Plants & Animal Derived Foods
Dairy Products like fortified breakfast cereal
Calcium bioavailability
Influenced by presence of anti-nutrients(decreases absorption low as 5%)
Oxalic Acid - spinach, rhubarb, swiss chard, & beet greens
Phytic Acid - wheat bran, legumes, seeds, nuts, & soy isolates
Functions of Calcium(5)
Skeletal system Bone formation
Muscle contraction
Cell signaling
Blood Clot Formation
Blood Pressure regulation
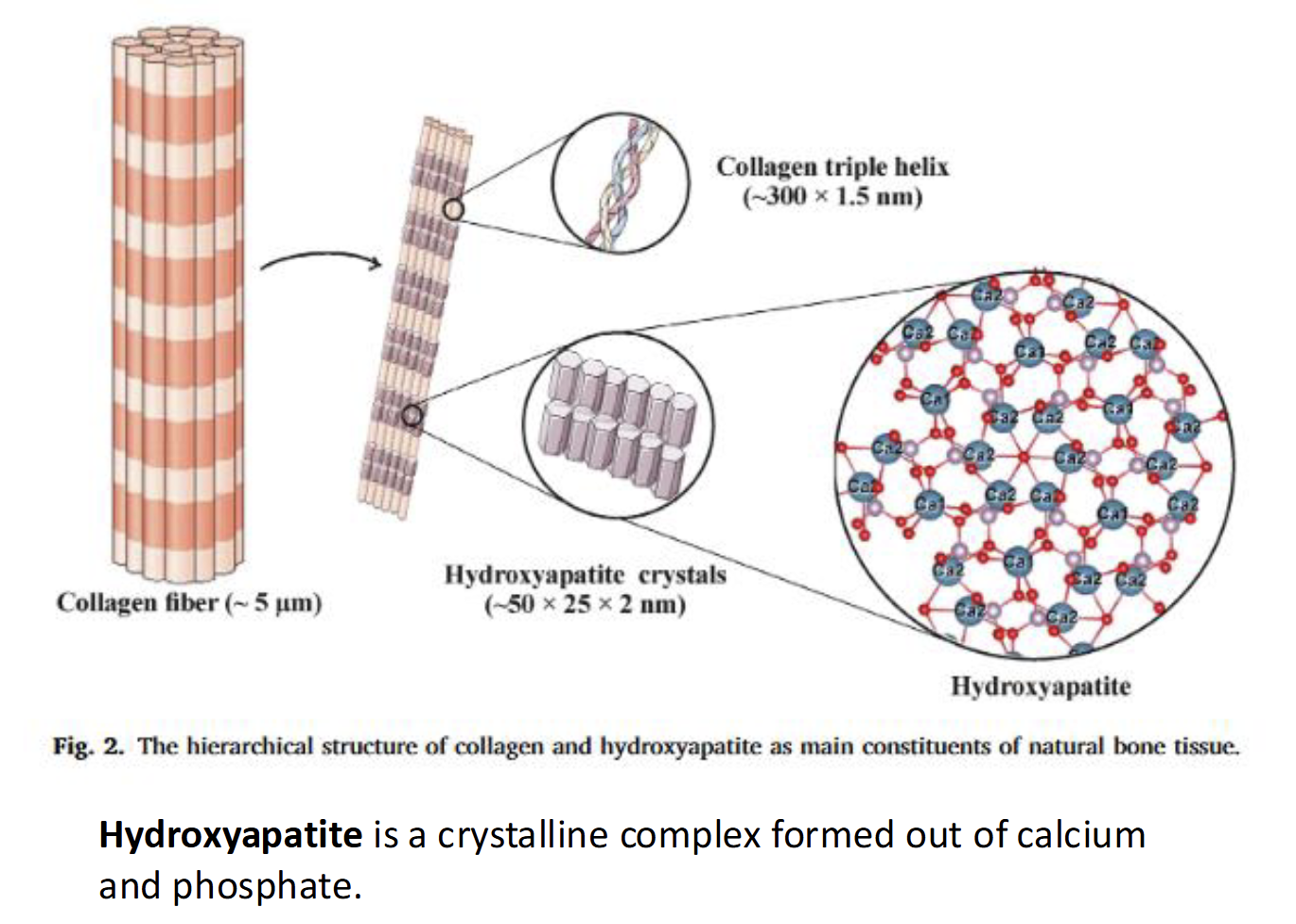
Structural function of Calcium for Bone Formation
As bone begin to form, calcium salts form hydroxyapatite crystals on protein collagen matrix which makes the crystals denser and stronger
Key players in Bone remodeling
Osteoblasts - bone building(formation) cells
Osteoclasts - bone breakdown or resorption cells
Osteocytes - mature bone cells (25 year half life)
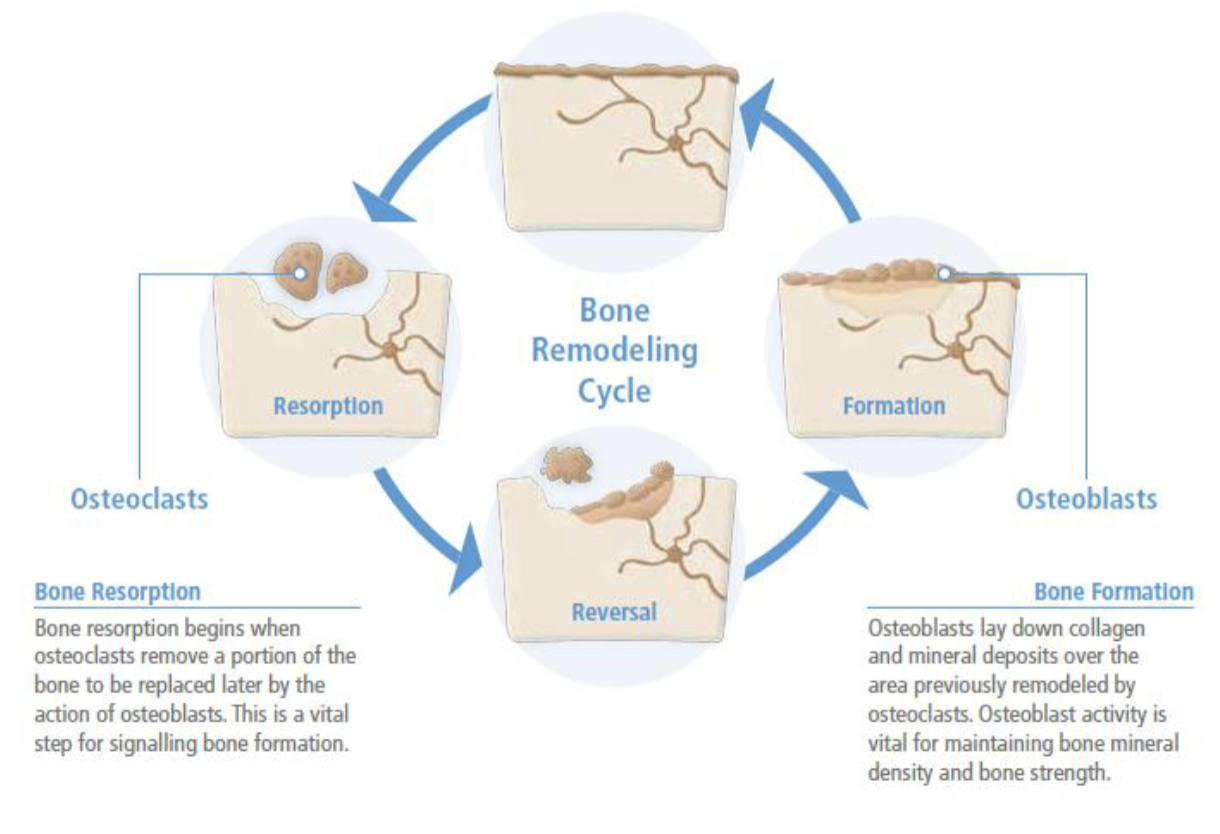
In terms of bone remodeling what is osteoporosis
Bone resorption(breakdown) > bone formation
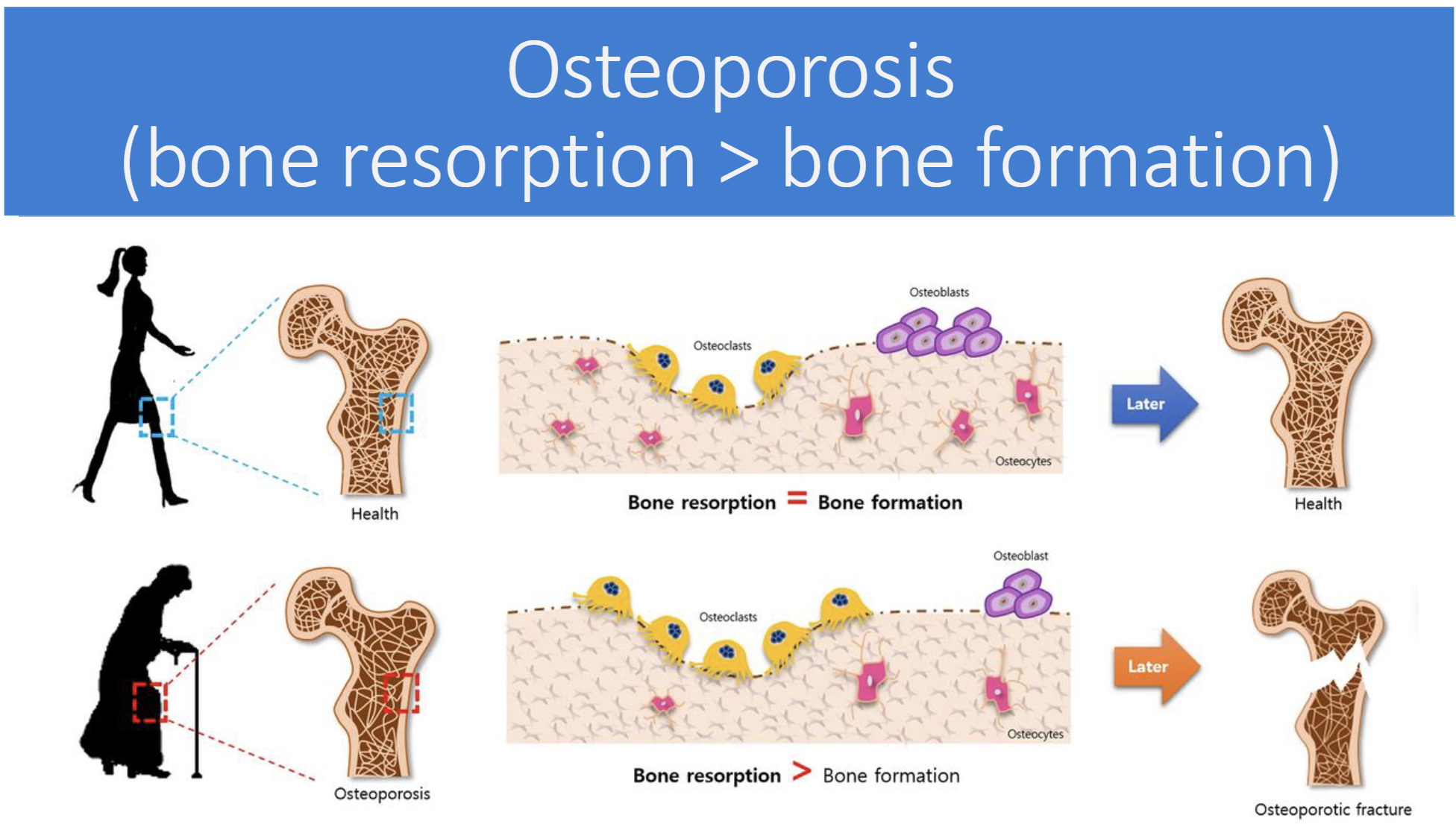
Risk factors for Osteoporosis(5)
Early menopause = low estrogen
Family hisotry of osteoporosis
Eating disorders
Early malnutrition
Vitamin D deficiency
RDA & UL of Calcium
RDA
Men: 1000mg/day
Women: 1000mg/day
UL
2500 mg/day
Excessive results in constipation, increased risk of urinary stones, kidney dysfunction, interference with absorption of other minerals
3 type of Vitamin K(quinone family)
K1: phylloquinone (plant foods)
K2(or MK): menaquinone (animal product, fermented foods, or by intestinal bacteria)
K3: menadione (synthetic forms used in animal feeds; humans don’t use)
Function of Vitamin K
Bone mineralization (osteocalcin(bone protein) synthesis)
improves osteoblast functions
inhibitory effect on bone breakdown(resorption) by apoptosis of osteoclast
Blood Clotting
Food Sources of Vitamin K
Dark leafy greens
RDA & UL of Vitamin K
RDA
Women - 90 micrograms/day
Men - 120 micrograms/day
UL
No UL
What might cause deficiency of Vitamin K(rare)
Antibiotics = killing gut micrbiome therby decreasing production
Fat malabsorption syndromes
Infants given vitamin K injejections at birth
How is Dietary Vitamin K absorbed in the S.I
Same Mechanism as dietary fat
(Lipolysis-Bile-esterification-lymphatic system- chylomicrons)
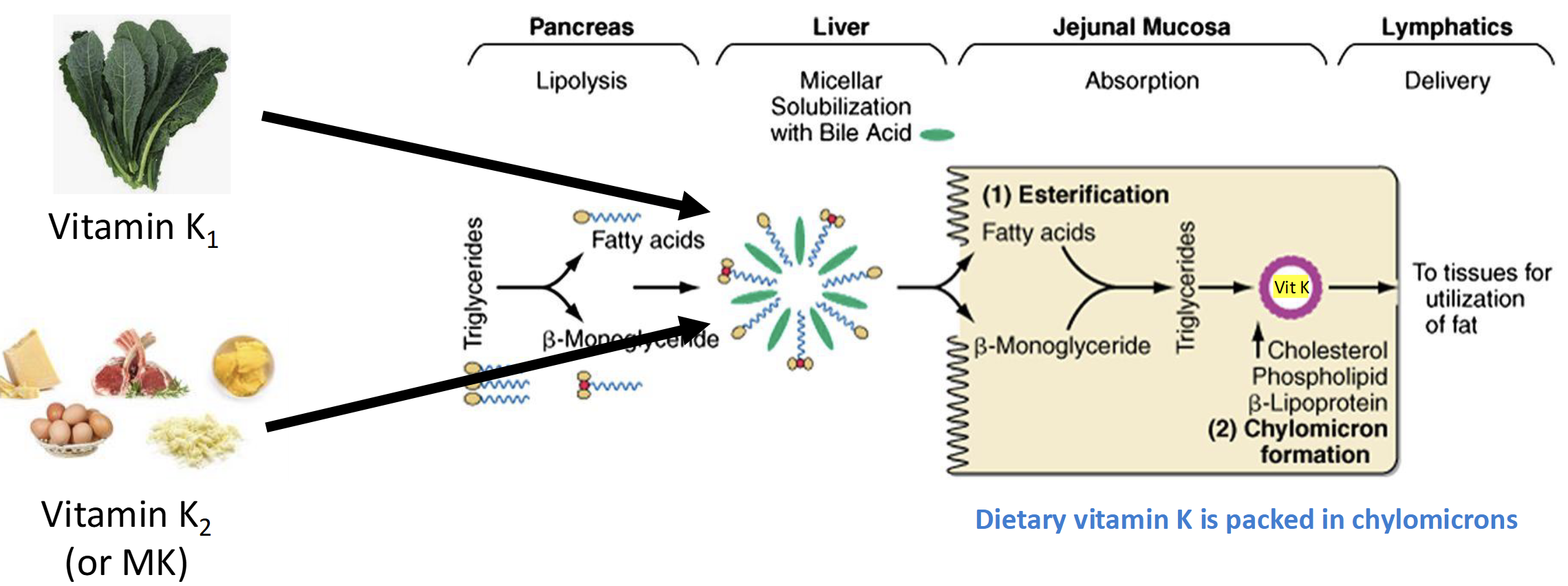
Bioavailability of Vitmin K
Antibioitcs & Coumadin(warfarin)
Micronutrients for Blood Health
Vitamin K, Vitamin B12 (cobalamin), Vitamin B9 (folate), Iron
Vitamin K in Blood Clotting
Vitamin K acts as a coenzyme that activates clotting factors which convert prothrombin to thrombin
Thrombin converts fibrinogen to firbin which is the insolble protein that forms clots
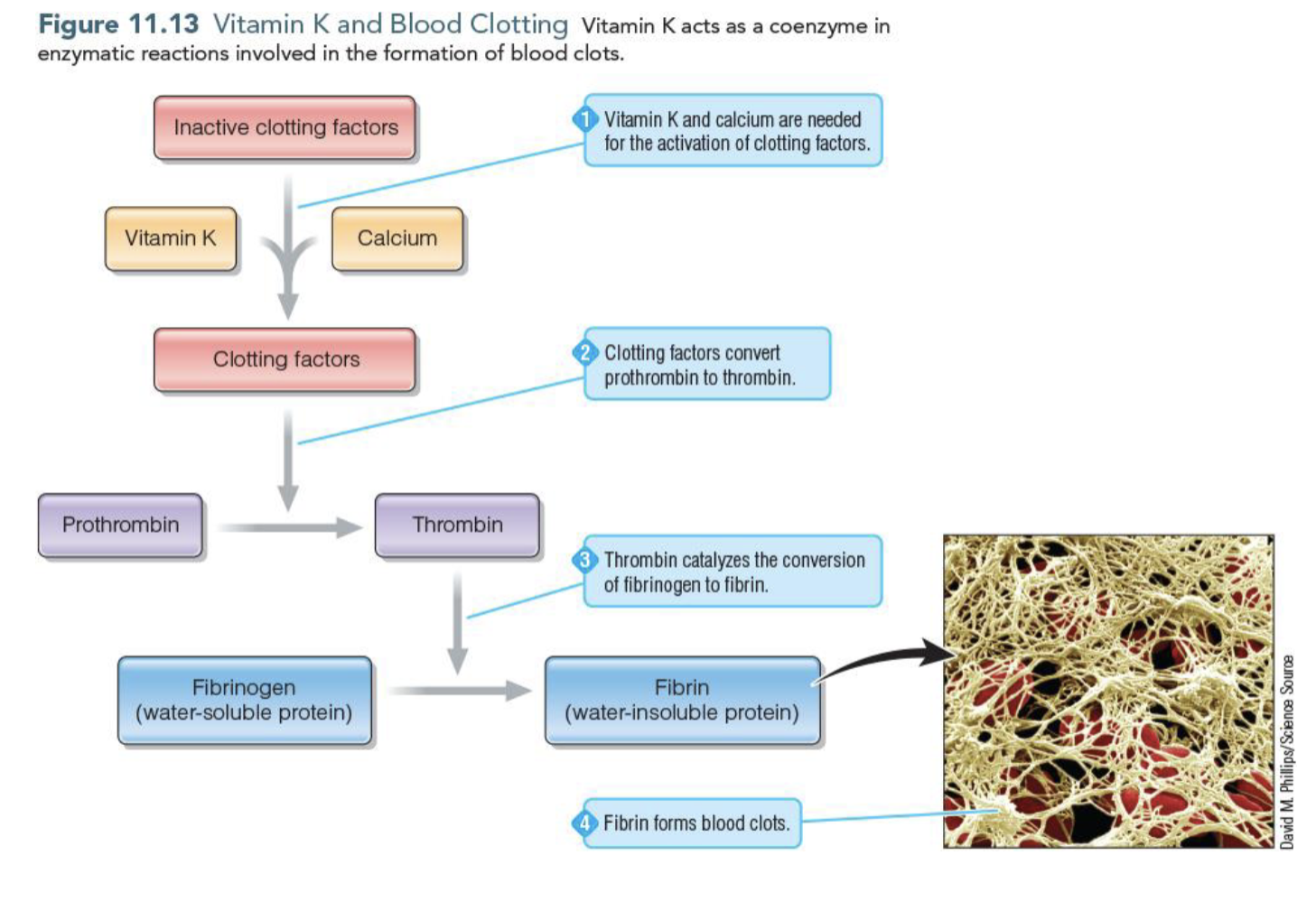
What is coagulation
The rapid blood clotting of an unjured blood vessel to stop the bleeding
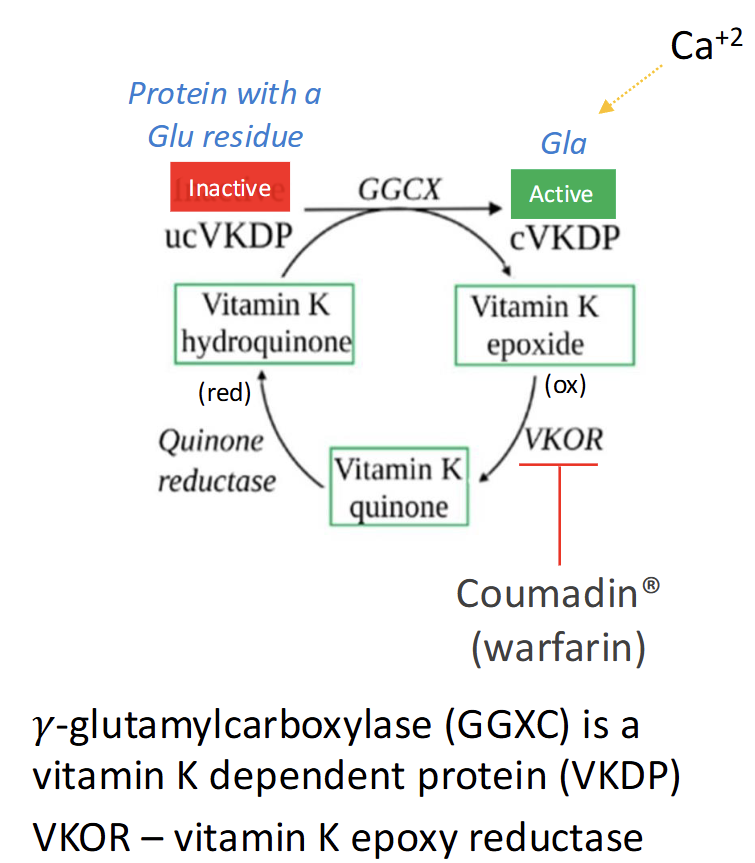
Explain this cycle
Vitamin K hydroquinone (active form) is used by γ-glutamylcarboxylase (GGCX) to activate vitamin K-dependent proteins (VKDPs) by converting Glu → Gla residues.
This reaction produces vitamin K epoxide (inactive form).
VKOR (vitamin K epoxide reductase) recycles vitamin K epoxide back to hydroquinone.
Quinone reductase converts dietary vitamin K quinone into hydroquinone.
Warfarin (Coumadin) inhibits VKOR, blocking recycling and reducing VKDP activation → anticoagulant effect.
What are Folate (Vitamin B9, Folic Acid)
Folate - naturally occuring form of B9
Folic Acid - synthetic form of B9 found in supplements & fortified foods
Folic acid is more bioavailable than folate
In the body, what happen to folate
Folate is converted to tetrahydrofolate(THF), a coenzyme form that accepts one carbon units at positions 5,10, or both
Food soucres of Folate
Cereal(Fortified) with more folic acid
Factors affecting Folate amount in food
Cooked foods have less folate than raw foods
Folate is senstive to heat, light, & oxygen
RDA & UL of Folate
RDA
Women: 400 microgram/day DFE
Men: 400 microgram/day DFE
DFE = unit of mreasure to describe the amount of bioavailable folate in food
UL = 1000 microgram/day
Role of Folate in one carbon transfer reactions(4)
Regeneration of THF
DNA syntehsis & repair
synthesis of building blocks of DNA and RNA(purines & pyrimidines)
Neural Tube development
methylation as key regulator of gene expression
Amino Acid metabolism
Hcy to Met conversion(also involes B12)
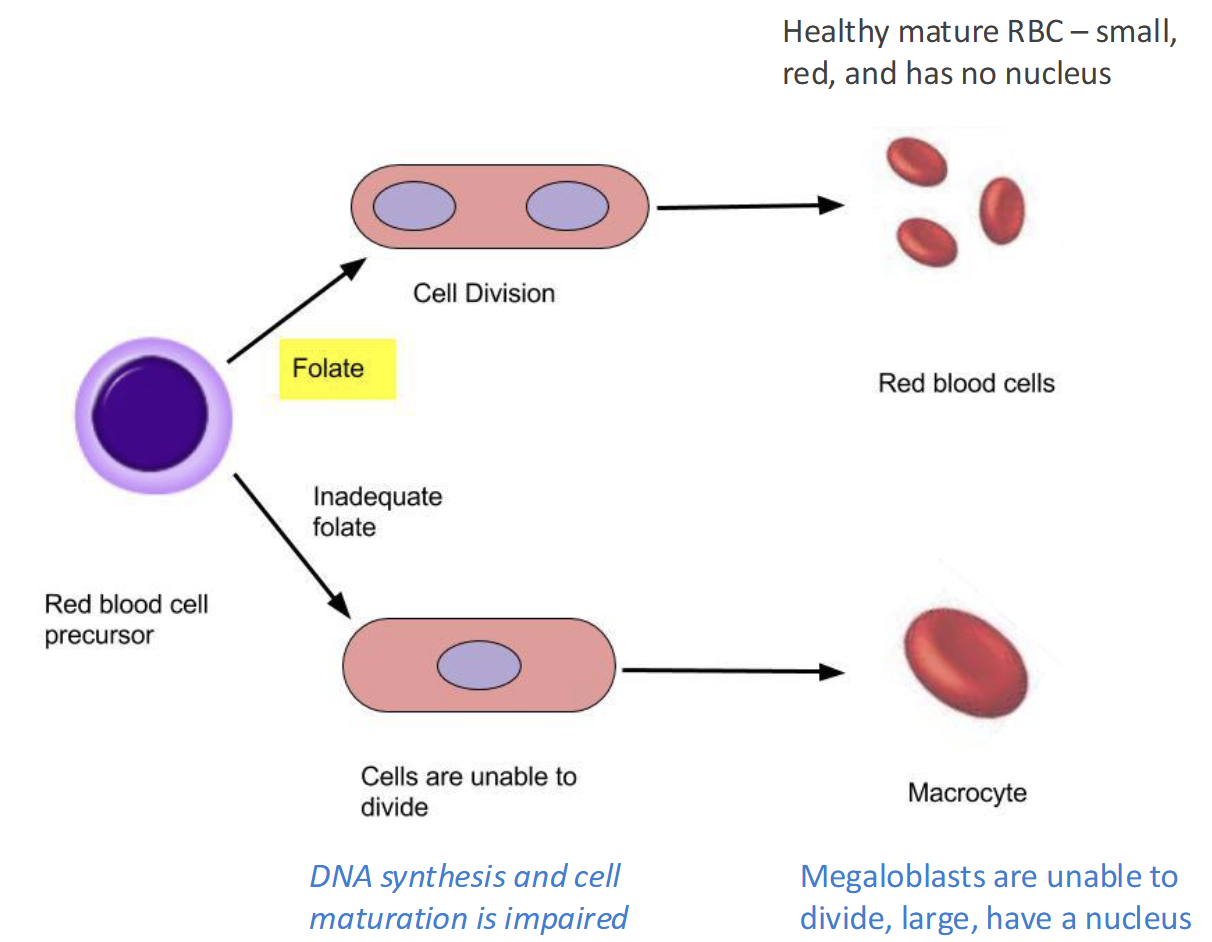
Folate Deficiency(RARE) causes
Lead to Megaloblastic macrocytic anemia(lack of O2)
Fatigue, weakness, headaches, pallor, SOB
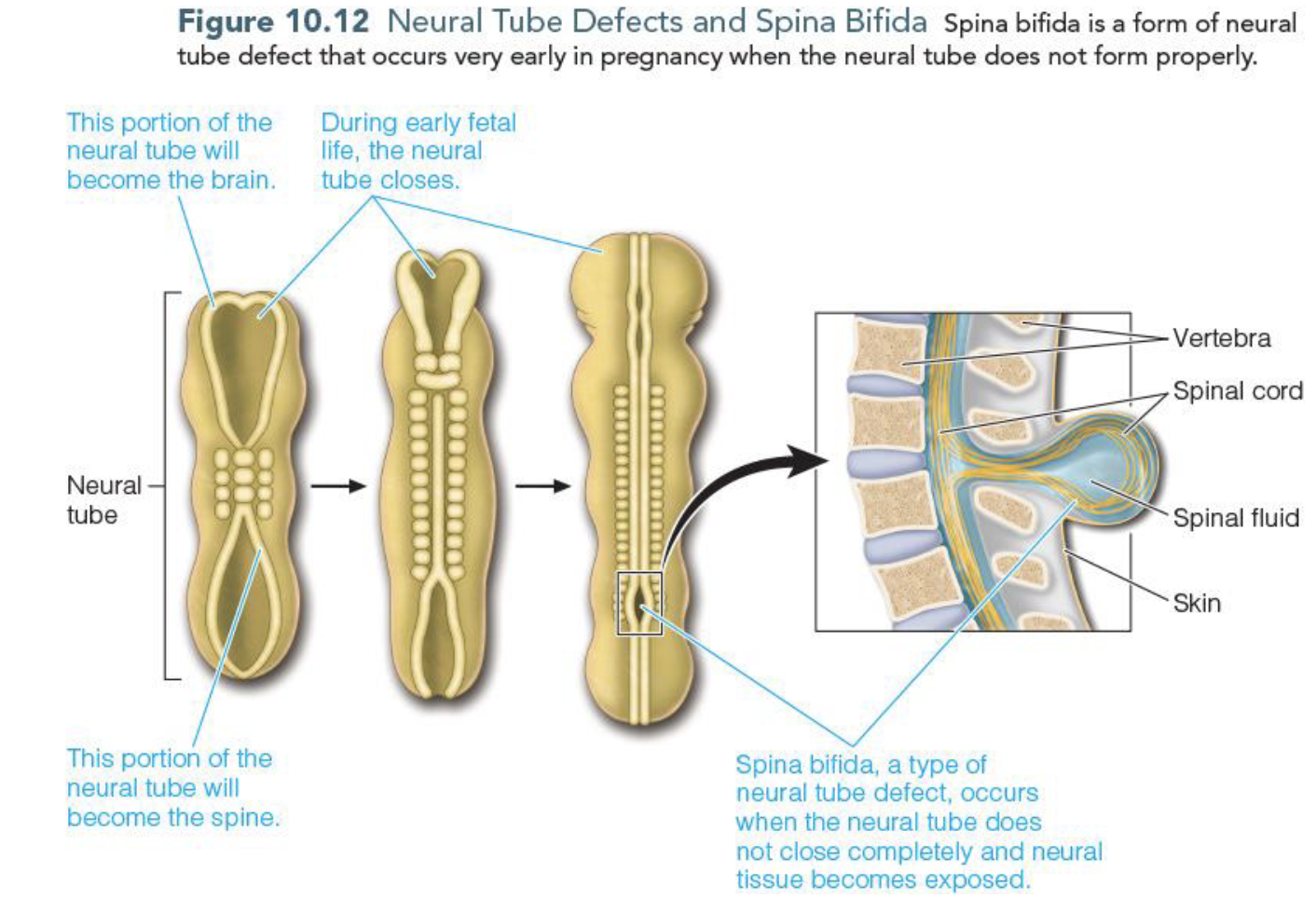
Folate & Neural Tube Defects
Folate is important in the formation of neural tube and w/o folate neural tube will have improper closing leading to
spina bifida (most common)/birth defect
Anencepahly/birth defect & fatal
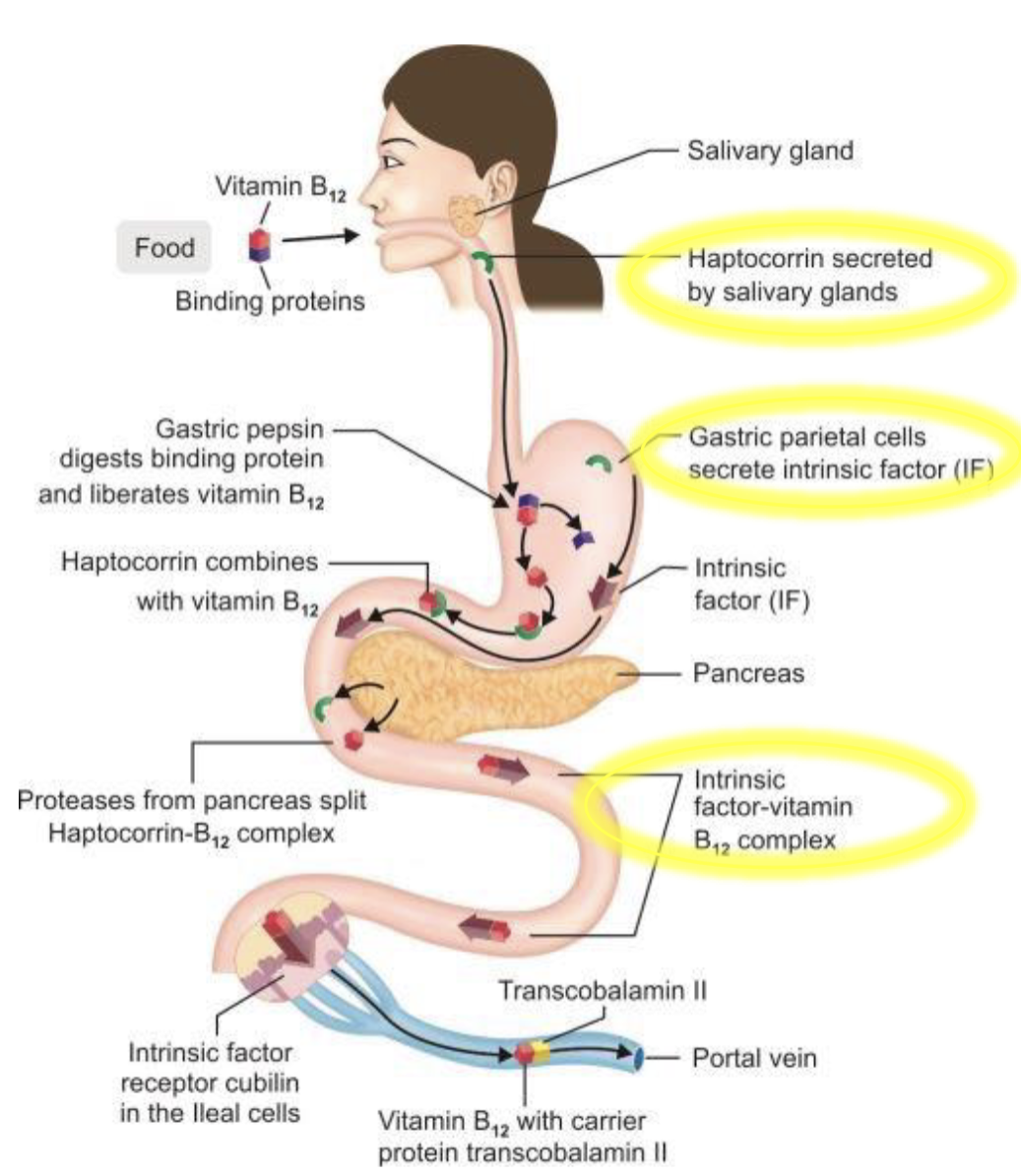
Vitamin B12 (Cobalamin) production & absorption
Vitamin B12 is only produced by microorganism & humans obtain it byconsuming animal based products
Absorption is assited by Haptocorin-B12 which protects from stomach acid, Intrinsic factors(IF) made by parietal cells, and IF-B12(S.I) absorbed as a complex
Food Sources of Vitamin B12
Seafood(Clams) & animal product
RDA & UL for Vitamin B12
RDA
Men: 2.4 microgram/day
Women: 2.4 microgram/day
UL
None
What affects aborsption of vitamin B12
Mediciation for Ulcers/GERD - H2 blockers & proton pump inhibitor
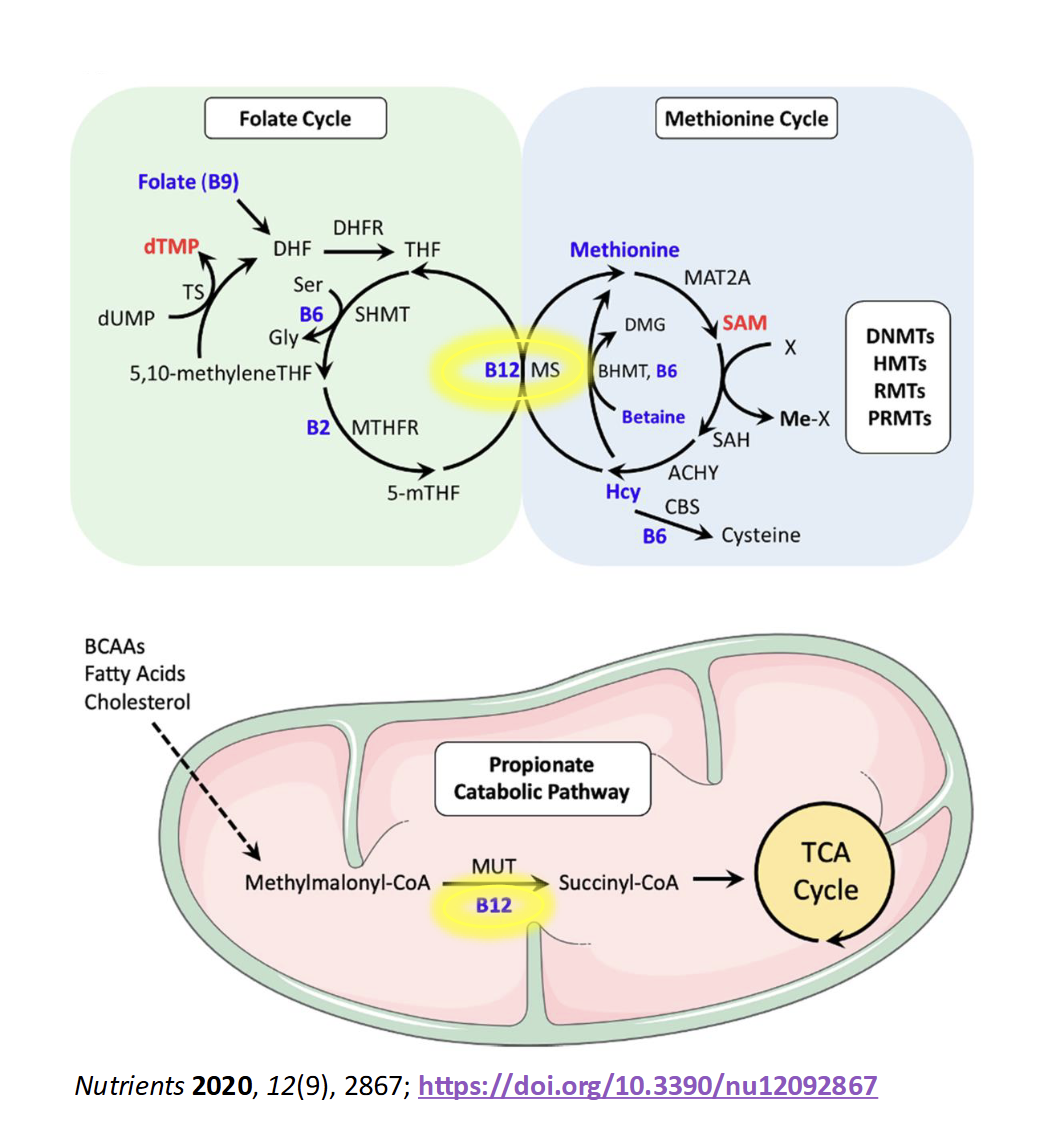
Functions of Vitamin B12(3)
ATP Production
TCA cycle, allows the body to use amino acid & FA for energy
Amino Acid Metaolism
conversion of homocysteine(Hcy) to methionine(Met)
Methylation(Me-X)
Met is used to create S-adenosylmethione(SAM), the bodys primary methyl donor
Regulation of gene expression
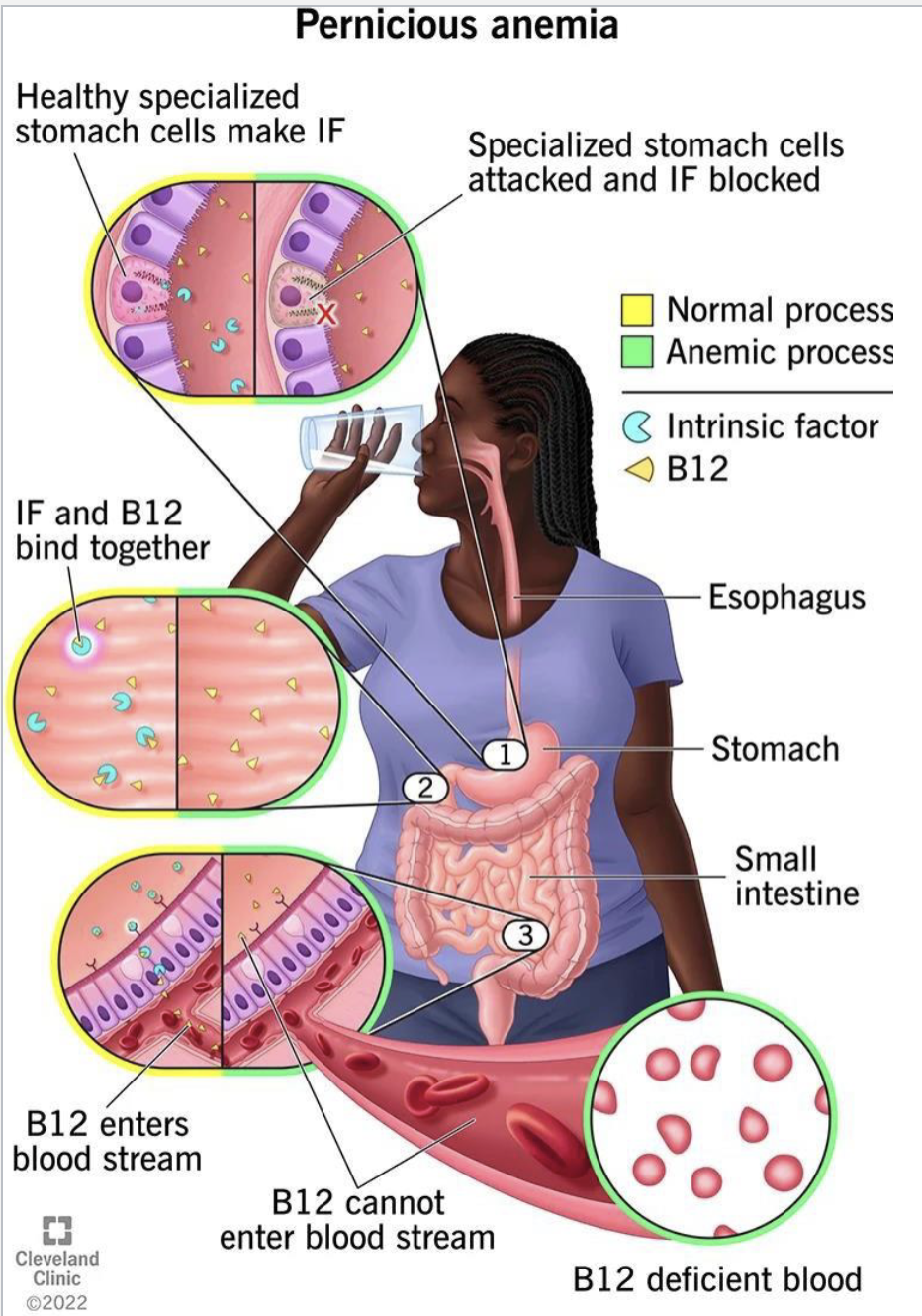
Vitamin B12 Deficiency(More poor absorption than inadequate intake) causes
Megaloblastic macrocytic anemia(lack of O2)
Pernicious anemia (autoimmume disease where antibodies destory intrinsic factors
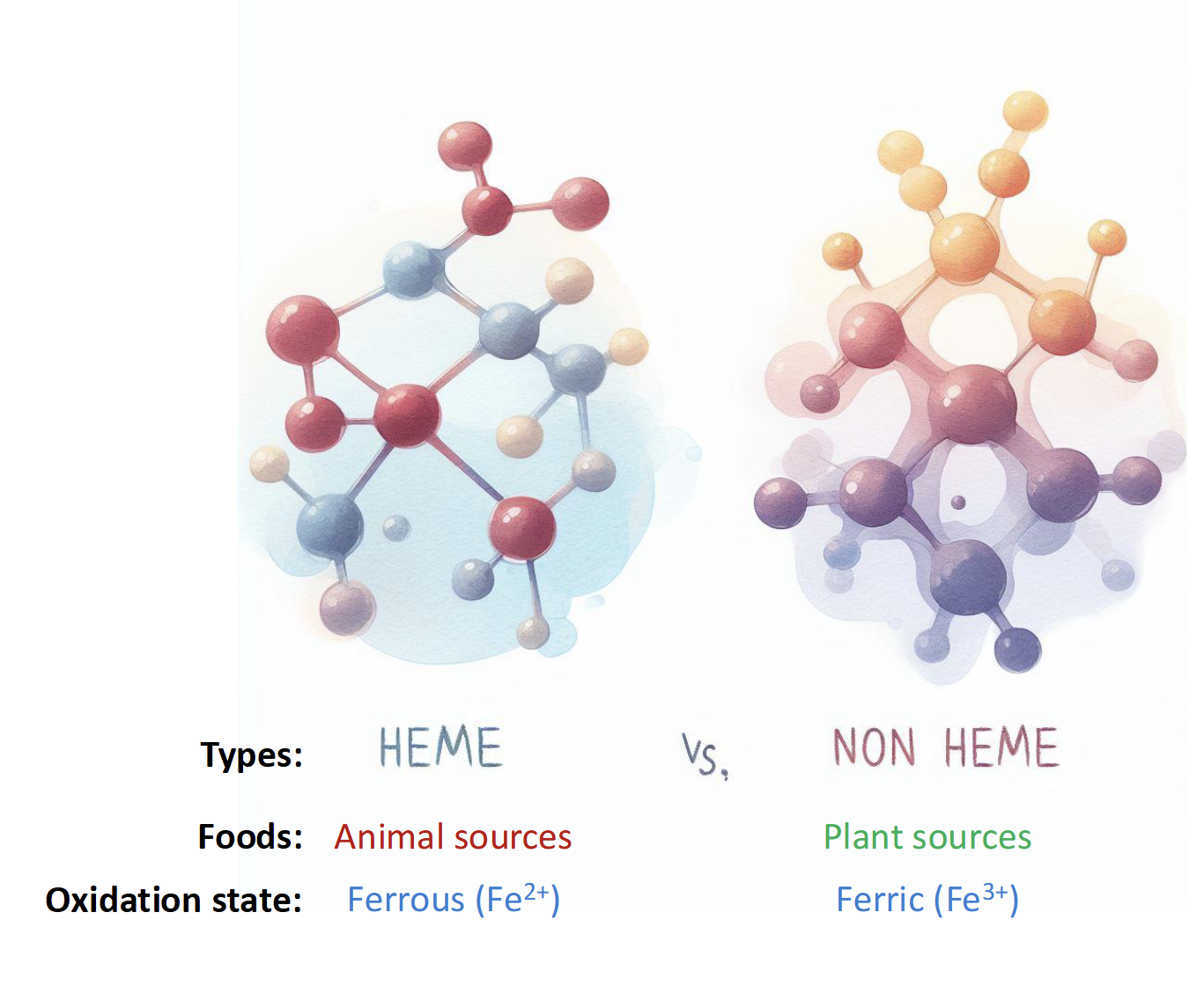
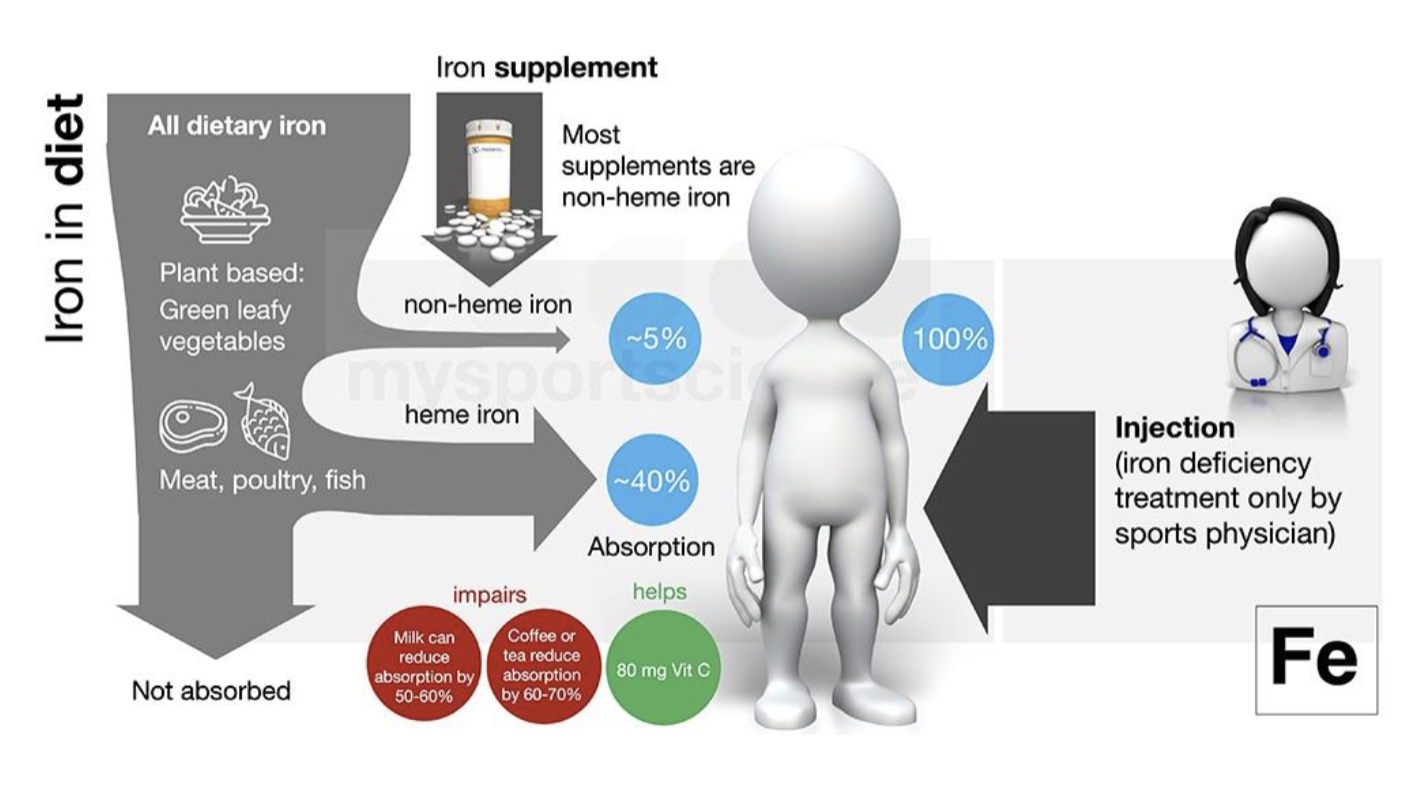
Types of Iron found in Food
Heme-Bound(animal)
15-40% absorbed
Non-Heme Bound(plants)
5-12% absorbed
NOTE: IRON Is poorly absorbed
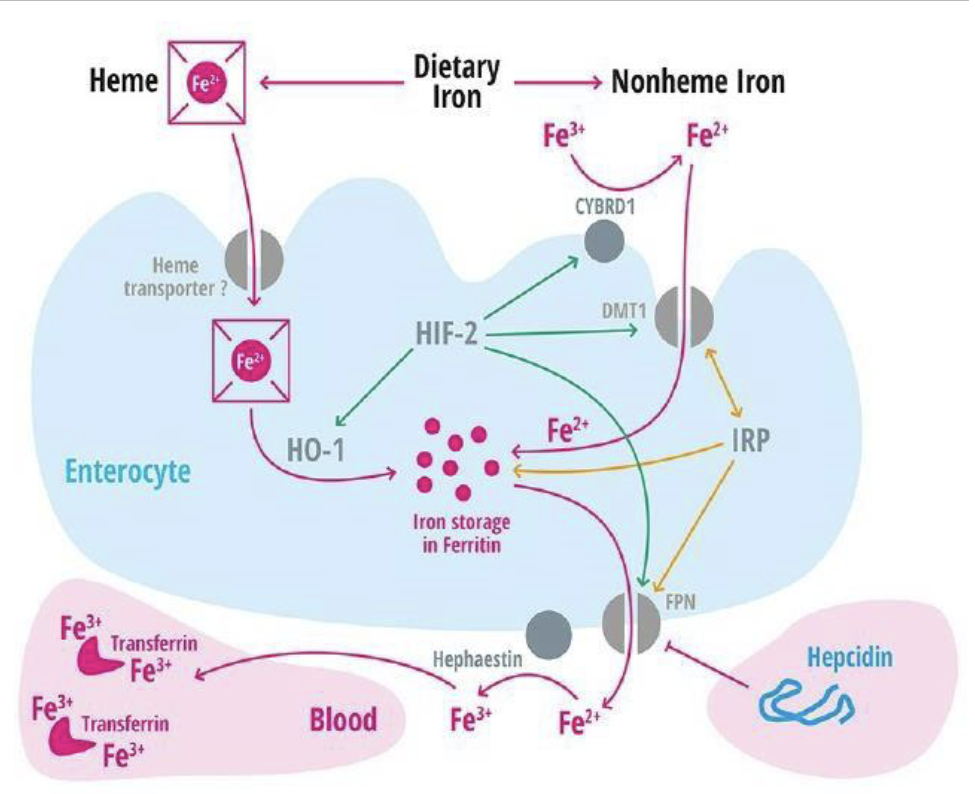
Absorption & Metabolism of Iron
Iron must be in Fe2+ state or bound to heme
Once in enterocyte, iron can be stored as ferritin or transported to circulation via ferroportin(FPN, a transmembrane transportor)
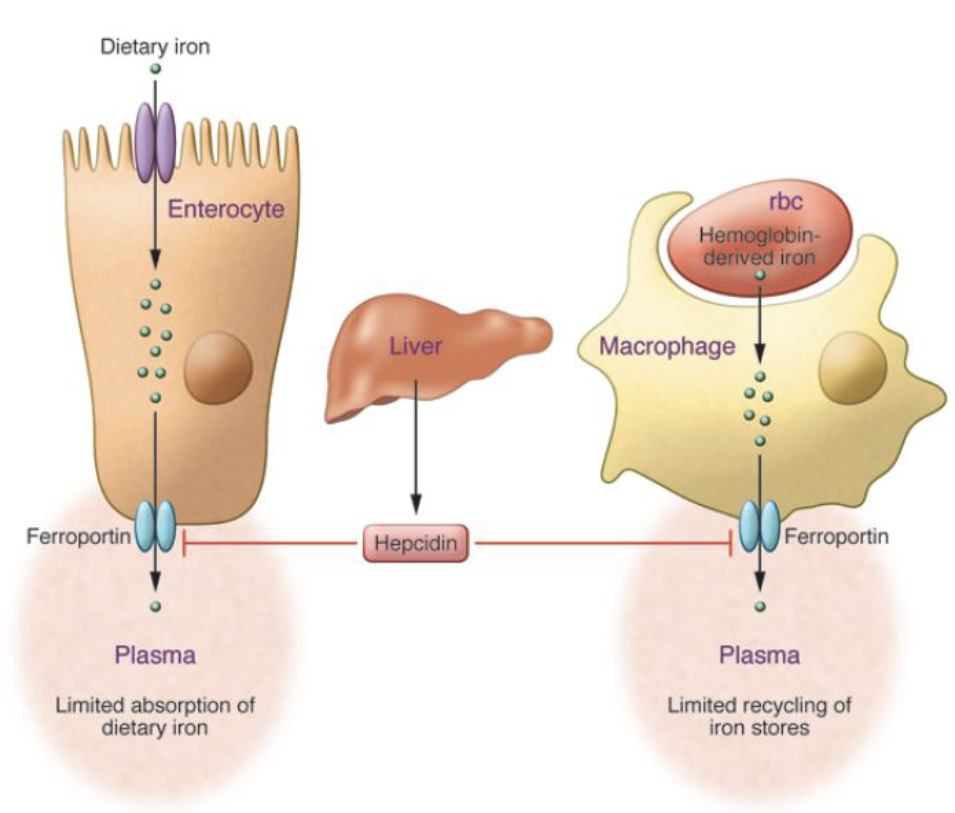
Hormonal Regulator of Iron Metabolism Conc.
High Iron Levels:
Hepcidin is released into circulation, where it binds to ferroportin, resulting in internalization & degradation of the transporter
Low Iron Levels
When Hepcidin levels are low, iron(Fe2+) can be released from enterocytes, where it’s oxidized into Fe3+ for binding to its protein carrier transferrin
Dietary compounds that inhibit/enhance iron absorption
Enhancers
Vitamin C
Meat factor
Inhibitors
Phytates(corn,whole grain, legumes)
Polyphenols(tea and coffee)
Oxalates(spinach, berries, chocolate)
Food Sources for Iron
Clams(Animal & Plant Product)
RDA & Ul for Iron
RDA
Women: 18mg/day
Men: 8mg/day
Ul
45 mg/day
Excessive lead to vomiting, diarrhea, constipation, and black stools
What is Hereditary Hemochromatosis
Genetic disorder that leads to greater iron absorption
Low levels of hepcidin