Unit 3- Bonding
1/81
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
82 Terms
Metallic Character of Elements
The weaker an atom attracts electrons (Zeff) the more metallic it is
Three categories for Metallic Character of the Elements
metals, nonmetals and metalloids
Metalloids
have properties somewhere between metals and nonmetals, though they are a generally bit more like nonmetals.
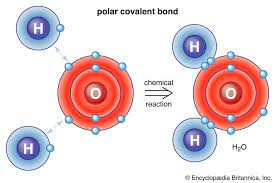
Covalent bonds
a chemical bond that involves the sharing of electrons to form electron pairs between atoms
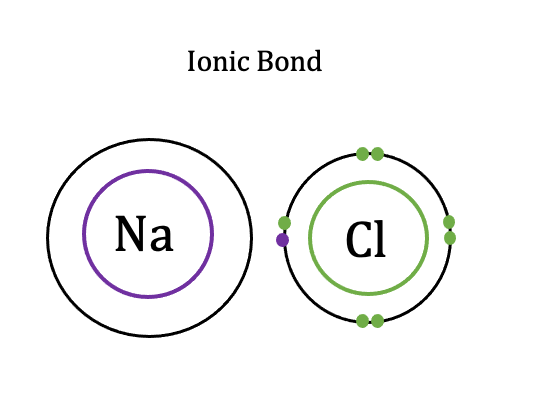
Ionic bonds
a type of chemical bond that occurs between two atoms when one atom transfers electrons to another atom. It is the electrostatic attractions between positive and negative ions
Ionic compounds are traditionally thought of as forming from the reaction of metals and nonmetals (though they form other ways as well)
cations
positive ions (removing electrons)
anions
negative ions (gaining electrons)
electrostatic attraction
attraction between positive and negative charges
Noble Gases and Bonding
only elements that exist as single, un-bonded atoms. This is due to their valence shell being full & stable.
Covalent Bonding Principles
electrons are shared between atoms to achieve a stable configuration
Covalent bonding usually results in atoms obtaining an octet of valence electrons through a combination of shared and unshared valence electrons (WITH EXCEPTIONS OF HYDROGEN, which only has 1 Principle energy level therefore only needing to complete shell with 2 electrons)
Bonding Pairs
shared electron pairs between atoms. Belongs to two or more atoms.
Non-bonding pairs/lone pairs
unshared pairs of valence electrons. Only belong to one atom.
ionic compounds
empirical formula where ratio is always the lowest whole number mole ratio of elements in the compound.
oxidation
an increase in oxidation state (charge) caused by the loss of electron(s)/electron ownership
reduction
a decrease in oxidation state (charge) caused by the gaining of electron(s)/electron ownership
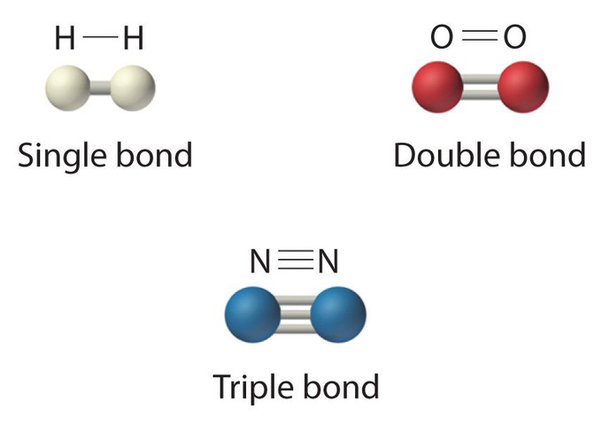
covalent triple & double bonds
only elements that exist as single, un-bonded atoms. This is due to their valence shell being full & stable.
Ionic Chemical Formulas & Names
Determining the formula of an ionic compound is done by balancing the ion charges so that the overall charge is neutral.
If the positive and negative charges on the ions are not equal, then subscripts are added to balance the charges. The “Criss Cross Apple Sauce” Method is a simple way to do this.
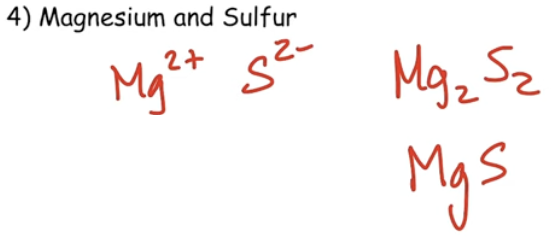
Bonding Pairs
shared electron pairs between atoms. Belongs to two or more atoms.
Polyatomic Ions
ions made up of more than one atom. These atoms are generally nonmetals and are covalently bonded together, but have an overall positive or negative charge and therefore act like ions.
They have their own specific formulas, charges and names that must be memorized.
Non-bonding pairs/lone pairs
unshared pairs of valence electrons. Only belong to one atom.
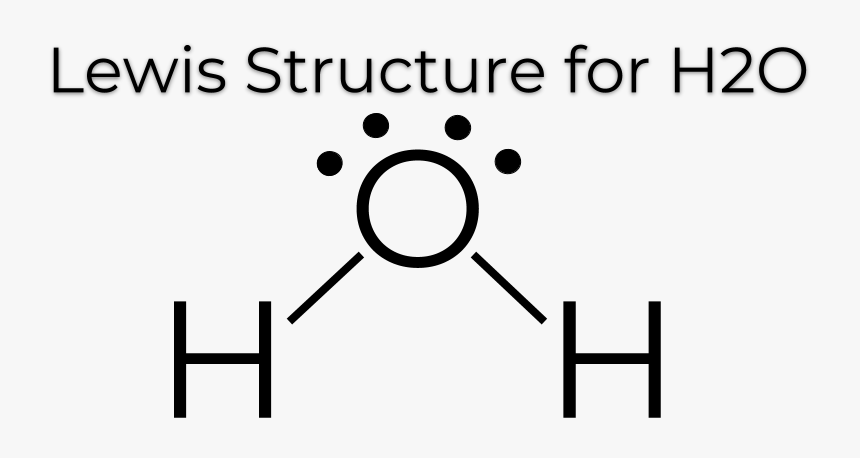
Lewis Dot Structures
simplified models for representing the covalent bonding between atoms, where each electron is represented by dots. Electrons are shown in pairs, with bonding pairs usually being represented as a line segment.
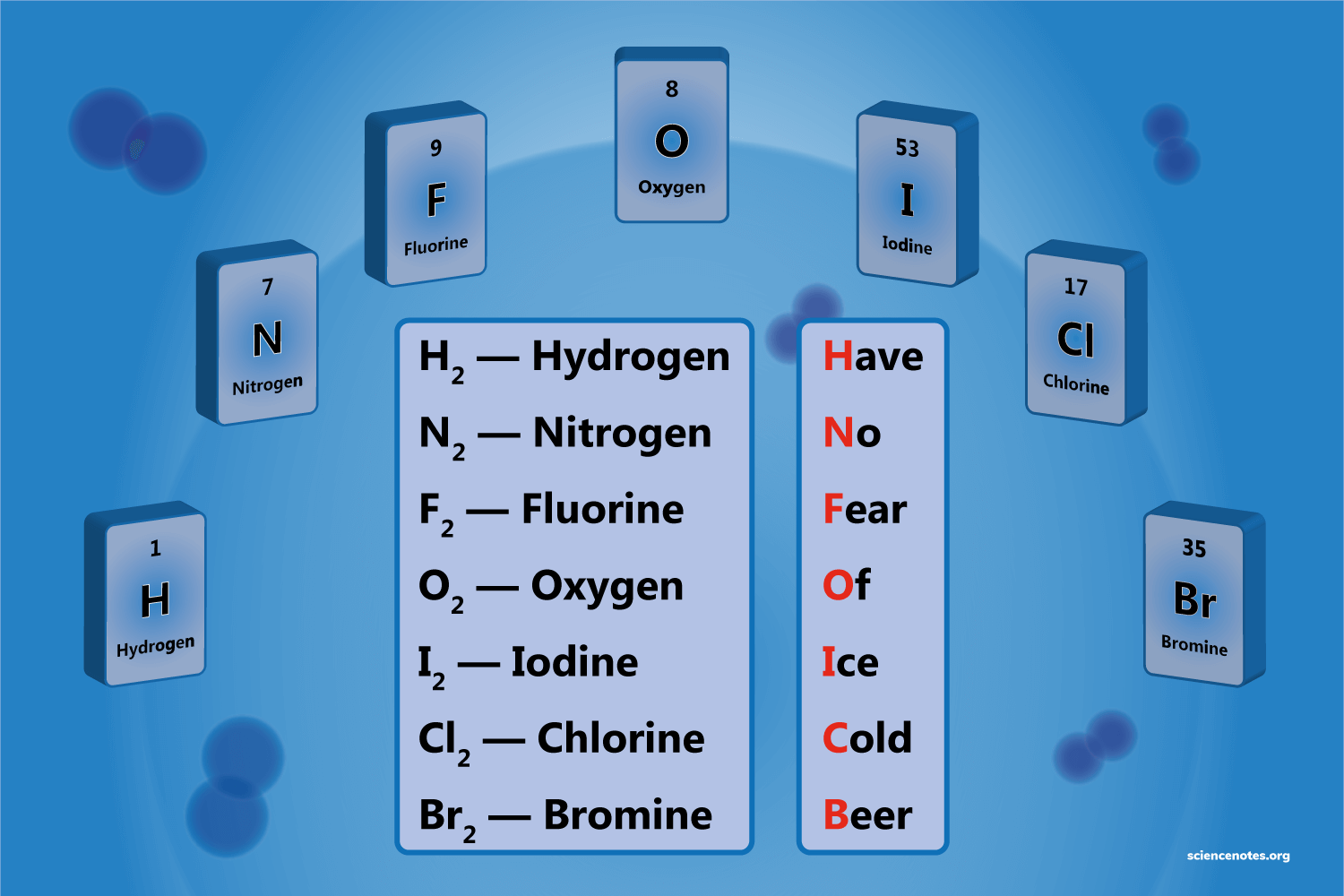
Diatomic elements
Diatomic elements are molecules composed of two atoms of the same element bonded together. Examples of diatomic elements include oxygen (O2), nitrogen (N2), and hydrogen (H2).
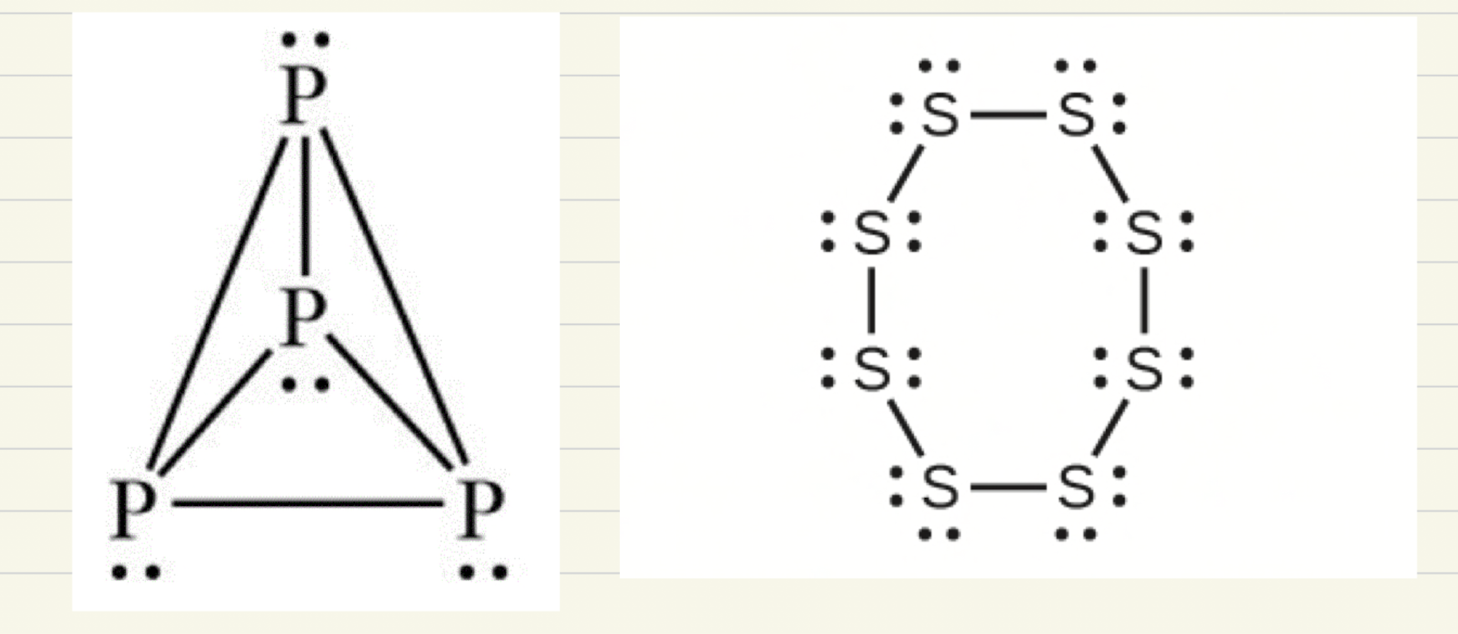
Lewis dot structures for phosphorus (P4) and Sulfur (S8)
Ionic Chemical Formulas & Names
Determining the formula of an ionic compound is done by balancing the ion charges so that the overall charge is neutral.
If the positive and negative charges on the ions are not equal, then subscripts are added to balance the charges. The “Criss Cross Apple Sauce” Method is a simple way to do this.
Rules for Naming Simple Ionic Compounds
Name both ions, cation (atom with positive charge) first.
Normal metal cations are named like the element: calcium → calcium
Normal non-metal anions are named with an “-ide” suffix:
chlorine → chloride
oxygen → oxide
hydrogen → hydride
nitrogen → nitride
Naming Ionic Compounds
Name the metal (the cation) as it appears on the Periodic Table.
For the non-metal (the anion) write the name on the Periodic Table and then replace the ending with ide.
CaCI2 = Calcium chlorine = Calcium chloride
AlN= Aluminum nitrogen = Aluminum nitride
Use the total charge on the non-metal (or polyatomic ion) find the charge on the transition metal.
Diatomic elements
Diatomic elements are molecules composed of two atoms of the same element bonded together. Examples of diatomic elements include oxygen (O2), nitrogen (N2), and hydrogen (H2).
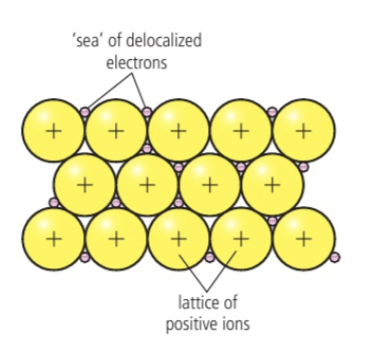
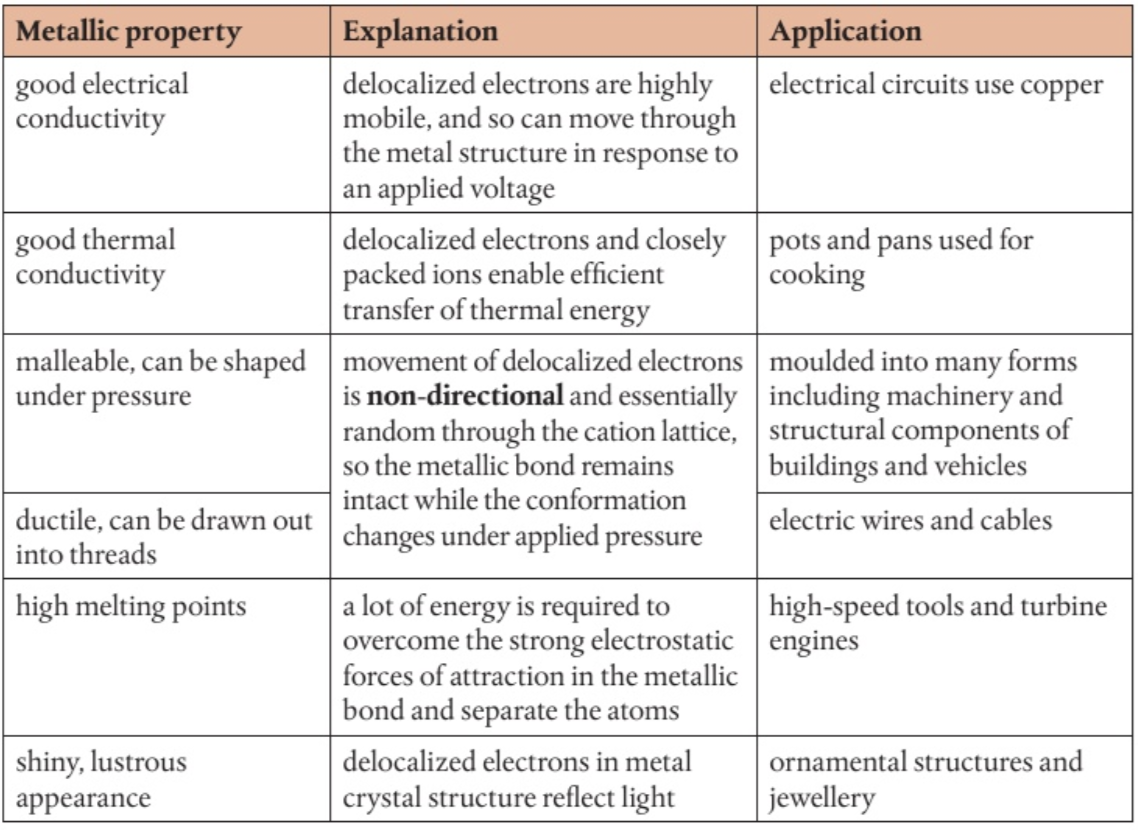
delocalised electrons & metallic bonding
In a molecule, ion, or solid metal, electrons that are not associated with a single atom or covalent bond are referred to as "free electrons". In the elemental state, when there are no other elements present to accept the electrons and form an ionic compound, the outer electrons are loosely held by the metal atom's nucleus and have a tendency to move around. These free electrons are not fixed in one position and can spread themselves throughout the metal structure. This allows them to move freely within a regular lattice structure, forming cations in the process.
(think about it like a close neighbourhood of families where the children do not belong specifically to any one set of parents but are free to wonder between the homes, causing a close association between the families)
Characteristic physical properties of metals
good electrical conductivity (electrical circuits use copper)
good thermal conductivity (pots and pans used for cooking)
malleable, can be shaped under pressure (moulded into many forms including machinery and structural components of building and vehicles)
ductile, can be drawn out into threads (electric wires and cables)
high melting points (high speed tools and turbine engines)
shiny, lustrous appearance (ornamental structures and jewellery)
alloy
An alloy is a mixture of two or more metals or a metal and a non-metal. It has different properties than the individual elements. Alloys are commonly used in manufacturing to create materials with specific properties, such as increased strength or resistance to corrosion. Examples include brass, steel, and bronze.
strength of the metallic bond is determined by
the number of delocalided electrons
the charge on the cation
the radius of the cation
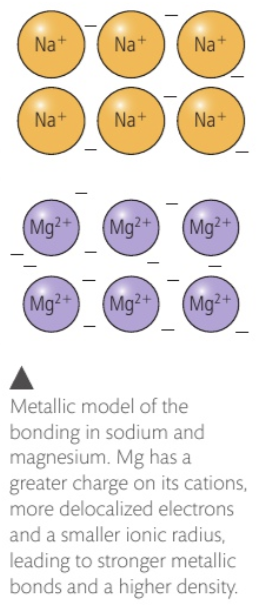
melting points of metals
the more delocalised electrons present and the smaller the radius of the atom, the higher the melting point of the metal.
strength of metallic bonding
decreases down a group as the size of the cation increasing, reducing the attraction between the delocalised electrons and the positively charged protons in the nucleus
metallic bonding of metals (trends)
Left to right across a period
Increasing melting point
greater attraction between ions and delocalised electrons
lower degree of reactivity
Down a group
decreasing melting point
weaker attraction between ions and delocalised electrons
higher degree of reactivity
Alloy structure
as an alloy mixture solidifies, ions of the different metals are scattered through the lattice, forming a structure of uniform composition.
Alloys contain metallic bonds as the delocalised electrons bind the lattice. The production of alloys is possible because of the non-directional nature of the delocalised electrons and the fact that the lattice can accommodate ions of different size.
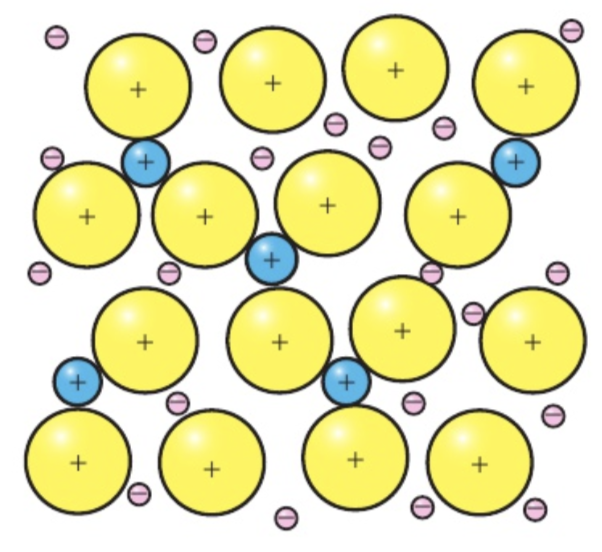
Alloy properties
they have properties that are distinct from their component elements due to the different packing of the cations in the lattice.
The regular arrangement of atoms in a pure metal is interrupted in the alloy by the presence of different cations , making it more difficult for atoms to slip over each other and so change the shape.
The alloy is often stronger, more chemically stable, and more resistant t corrosion than its component elements. For example, steel, which is an alloy of iron is 1000 times stronger than iron.
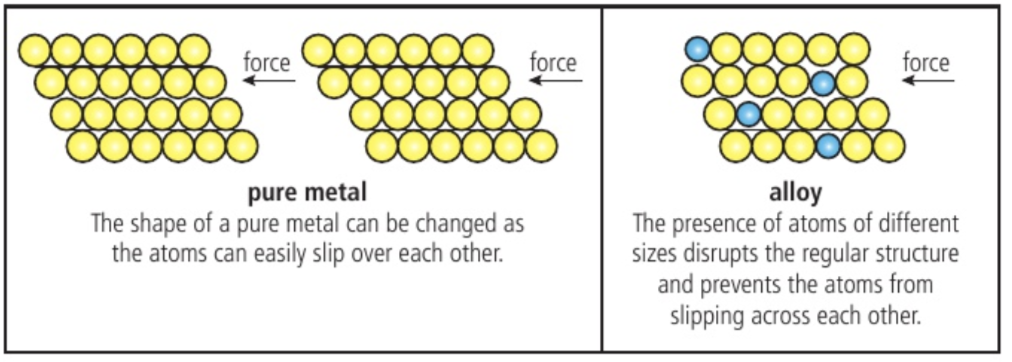
Common alloys
Alkanes
simplest class (family) of organic compounds. They are hydrocarbons in which the carbon atoms are held together by single bonds.
General formula: CnH2n+2
IUPAC Naming
Root/Parent Name: the number of carbon atoms in the longest continuous chain
Meth
Eth
Prop
But
Pent
Hex
Suffix: The class of compound
ane: alkane (no functional group)
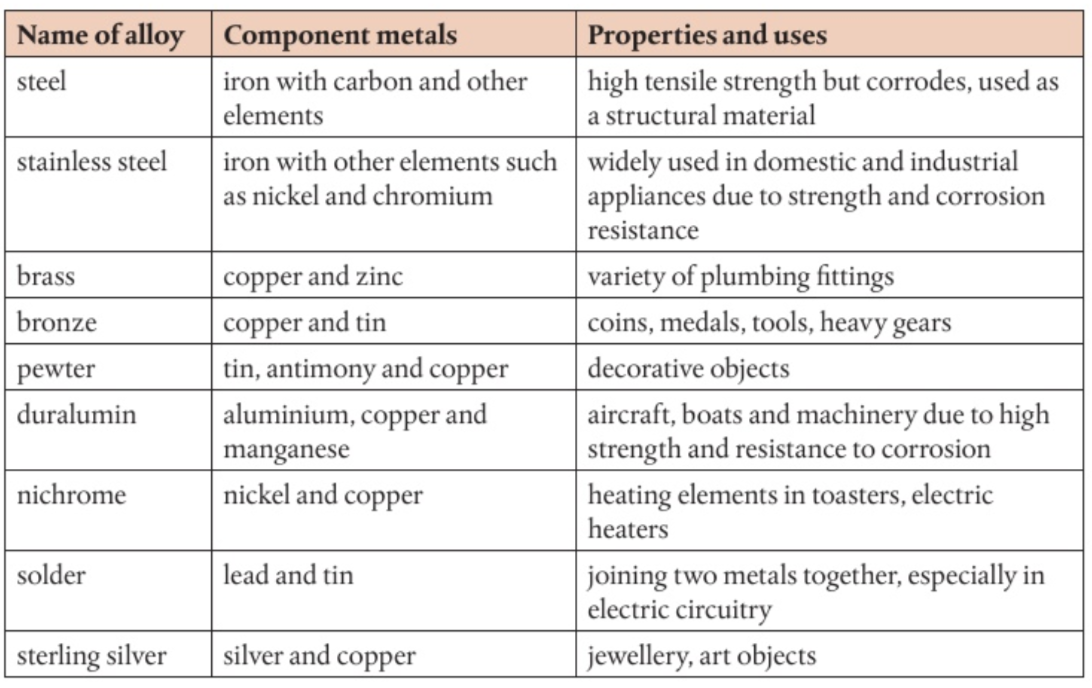
Alkenes
General formula: CnH2n
Functional Group: C=C
Naming Suffix: -ene
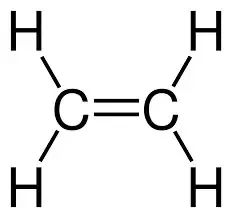
Alkynes
General formula: CnH2n-2
Functional Group: N/A
Naming Suffix: -yne
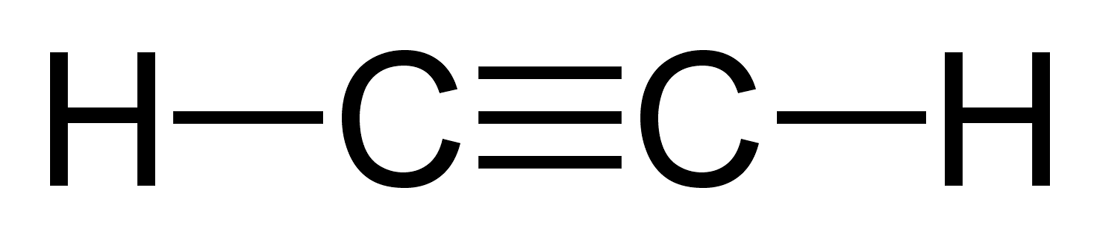
Alcohols
General formula: CnH2n+1OH
Functional Group: OH
Naming Suffix: -anol
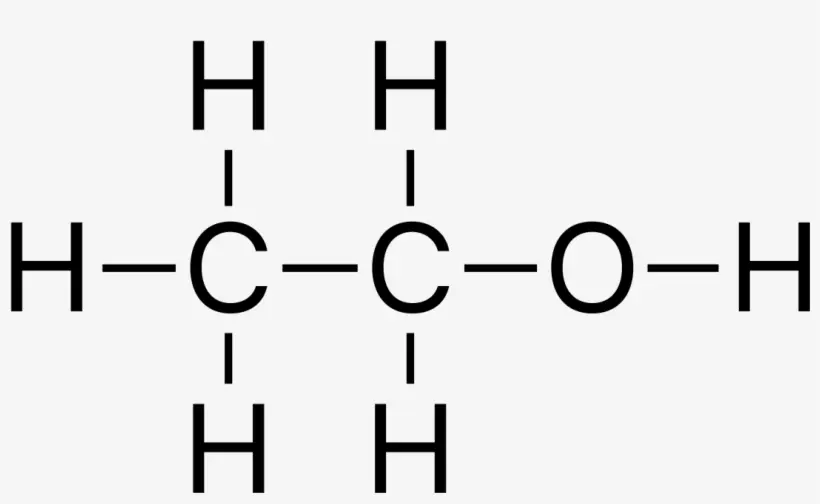
Lewis dot structures for phosphorus (P4) and Sulfur (S8)
Noble Gases and Bonding
double covalent bond is covalent bond formed by atoms that share two pairs of electrons.
triple covalent bond is a covalent bond formed by atoms that share three pairs of electrons
Bonding Pairs
shared electron pairs between atoms. Belongs to two or more atoms.
Non-bonding pairs/lone pairs
unshared pairs of valence electrons. Only belong to one atom.
Transition Metal
element with an incomplete d-sublevel (1-9 d electrons) in one or more of its oxidation states (Oxidation states can be more or less be thought of as charges the transition can have as ion or within compounds)
What metal in the d-block is not a transition metal?
Zinc, as it has a complete d-block configuration for all of its oxidation states
What is the only oxidation state of copper which allows it to be a transition metal?
When it forms a Cu2+ oxidation state- electron configuration is [Ar]3d9, meaning that it has an incomplete d-block configuration
Metallic bond strength & physical properties
Both d & s electrons can become delocalised and contribute to the metallic bonding in transition metals. This makes the metallic bonds in transition metals much stronger than those in typical s & p block metals. As a result, transition metals typically have much different properties than s & p block metals, listed below:
High electrical and thermal conductivity
High melting points
Malleable & Ductile
High tensile strength
Why does Zeff remain (almost) constant along transition elements?
Occurs due to the similar shielding effect of inner electrons. The inner electrons shield the outer electrons from the full positive charge of the nucleus. As we move across a period in the periodic table, the number of protons in the nucleus increases, which would suggest an increase in Zeff. However, the number of inner electrons also increases, providing more shielding and offsetting the increase in Zeff.
Transition Metals Important properties
Multiple oxidation states
Catalytic behaviour
Show Magnetism
Form a variety of complex ions
Form coloured compounds
High electrical conductivity (due to more delocalised electrons, causing increase of strength of metallic bonds)
High melting points (due to more delocalised electrons, causing increase of strength of metallic bonds)
Transition Metals and multiple oxidation states
Metals in group 1, 2, 3 form ions with only one charge
Every transition metal forms a +2 ion, which is due to it losing its 2 valence electrons (as all transition metals have s-orbitals as their valence electrons)
As the s-electrons and inner d-electrons are high in energy (due to convergence), it is possible for not just the outer s-electrons to be removed, but also the d-electrons. This explains how multiple oxidation states can be formed.
Naming with Transition metals
oxidation state must be included in the name as a roman numeral (oxidation number) (e.g Copper (II) oxide, CuO)
nonmetal in the compound will have its ending switched to ‘ide’, while metal will maintain its regular name (e.g Titanium (II) Oxide)
Transition Metals: Catalytic Behaviour
can act as Catalysts
Transition metals are particularly effective catalysts because they have the ability to change their oxidation state during a reaction, which allows them to participate in multiple steps of the reaction.
Catalysts
a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction.
Transition Metals: Magnetism
Spinning electric charges create magnetic fields. Each electron, therefore, creates its own very tiny magnetic field. With most substances, the electrons are arranged randomly and these magnetic fields cancel out, so there is no net magnetic field.
If subjected to an external magnetic field, the electrons within an element will align with, or against, the applied magnetic field. Depending on the electron configuration of an element, it will respond differently to these applied magnetic fields.
Magnetism is related to unpaired electrons
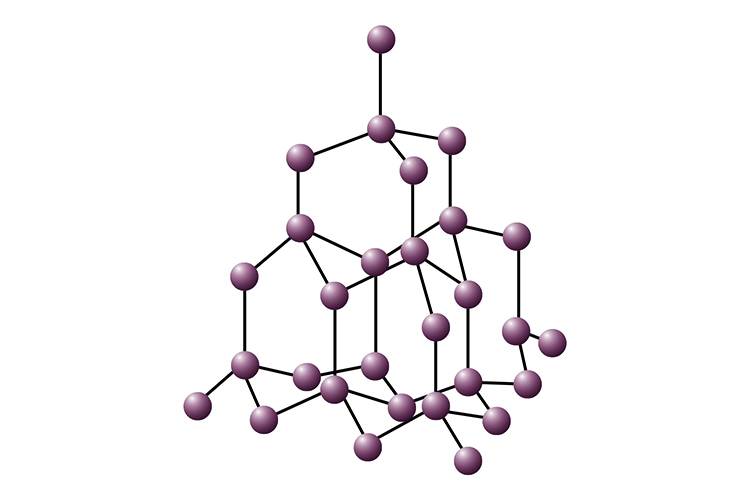
Giant Covalent Solids & Structures
while most covalently bonded substances are molecular, there are a few substances that form a different kind of structure called giant covalent structures.
always solids at room temperature
strongest bond
much harder that molecular solids
higher melting points than molecular solids
Typical example of a giant covalent solid is a diamond, which is made of carbon. Other examples are of silicon (Si) and silicon dioxide (SiO2)
Covalent Molecular Bonds
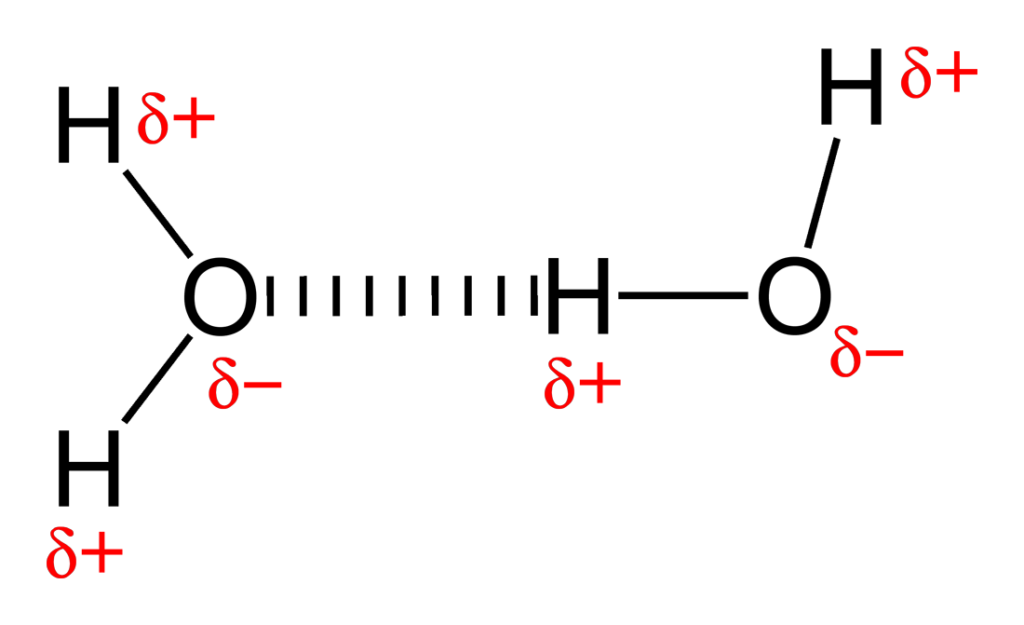
the sharing of electron pairs between atoms that form molecules, with weak intermolecular forces between the molecules.
can break apart easily when heated (low melting point)
forms weakest bond
Physical Properties of all Bonds
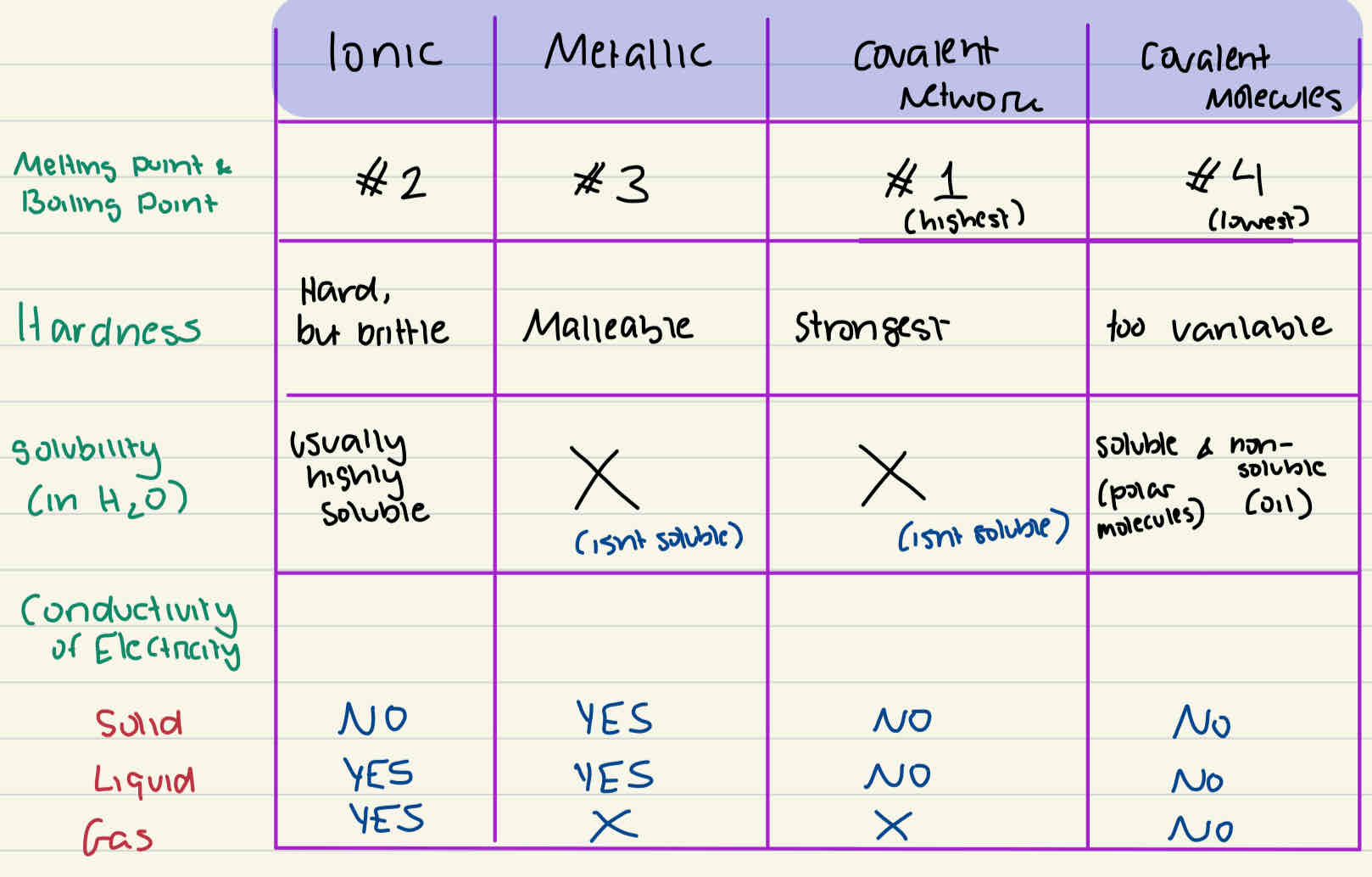
What is hardness determined by?
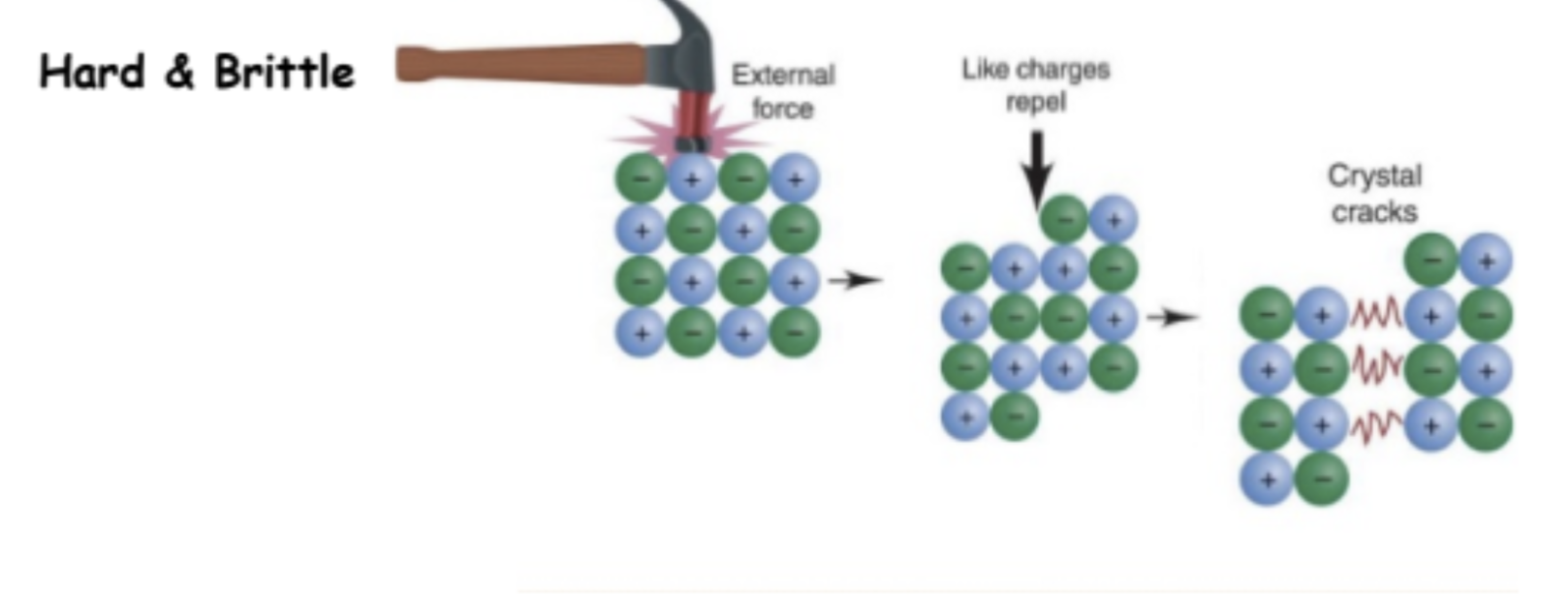
depends on strength of the attraction between neighbouring particles. Stronger inter particle attractions lead to solids that are harder, and in many cases, more brittle, meaning they tend to break-split apart rather than bend.
Weaker inter-particle attractions can lead to substances being softer solids. If attractions are weak enough than substances will be liquid or gas, and so the term “hardness” wouldn’t apply.
Malleability
the ability to be shaped, which is also linked to hardness of a metal
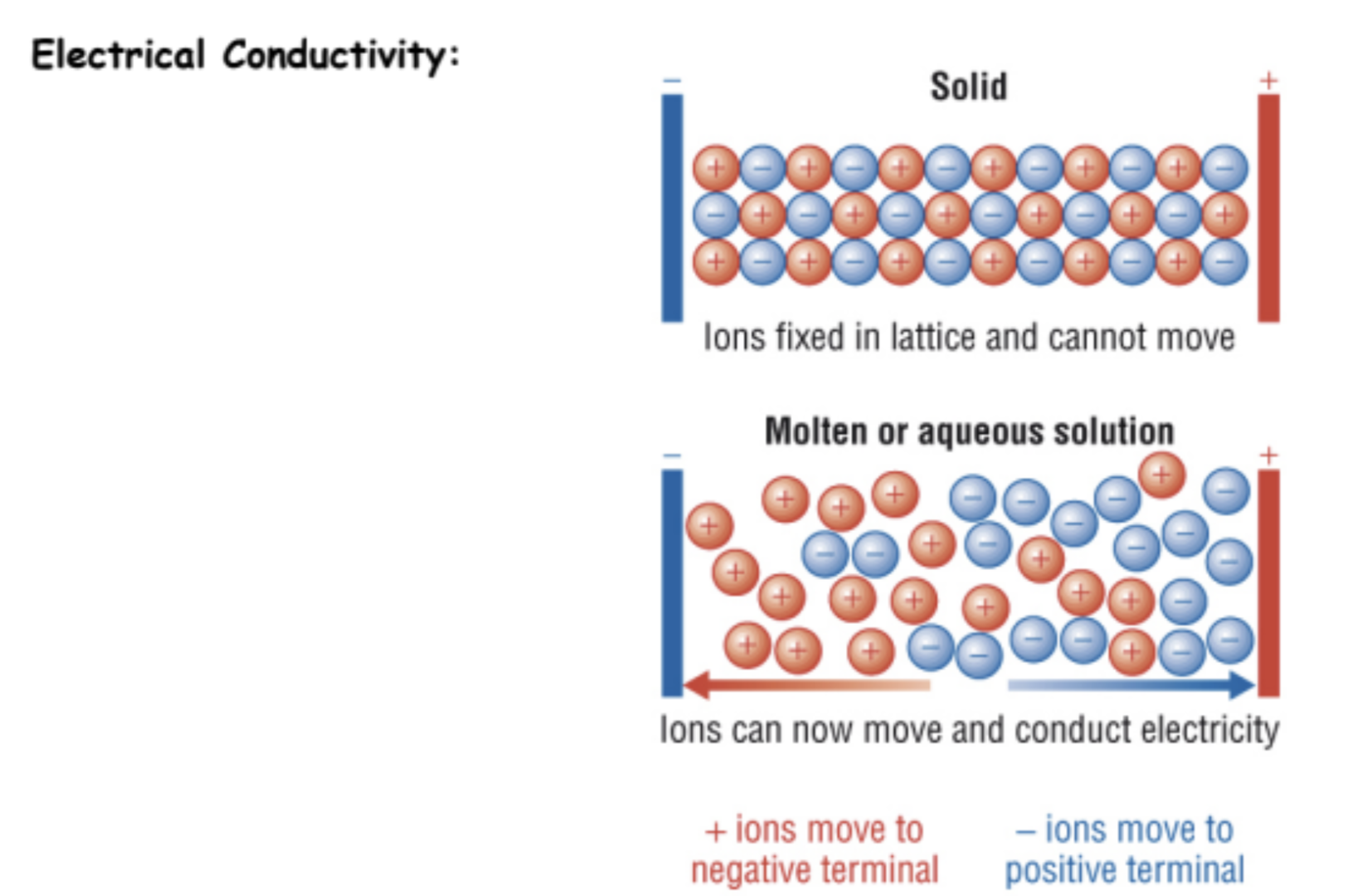
What is electrical conductivity determined by?
The ability to conduct electricity requires presence of free-moving charges, so that current (a flow of electrons) can occur in a circut.
What is Thermal Conductivity determined by?
based on how efficiently atoms can pass along thermal energy to one another. Atoms that are more tightly packed together tend to be better conductors of thermal energy, while molecular substances, especially gases, tend to do so less efficiently.
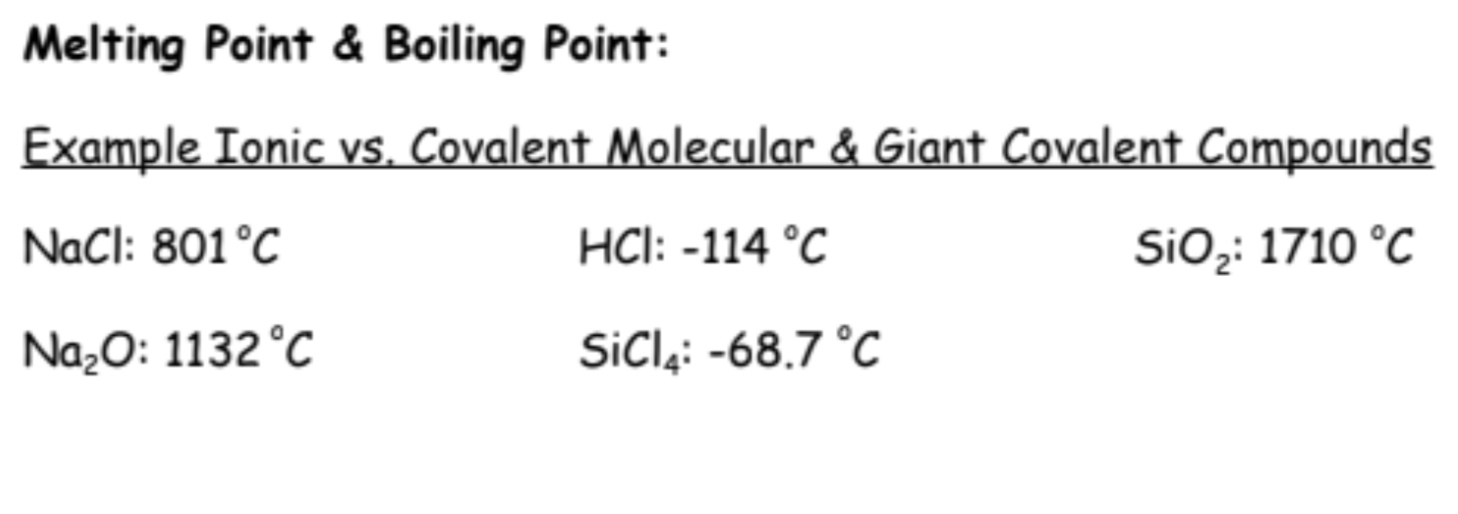
What is Melting & Boiling points determined by?
depend on the strength of the attraction between neighbouring particles. Stronger inter-particle attractions lead to higher melting and boiling points
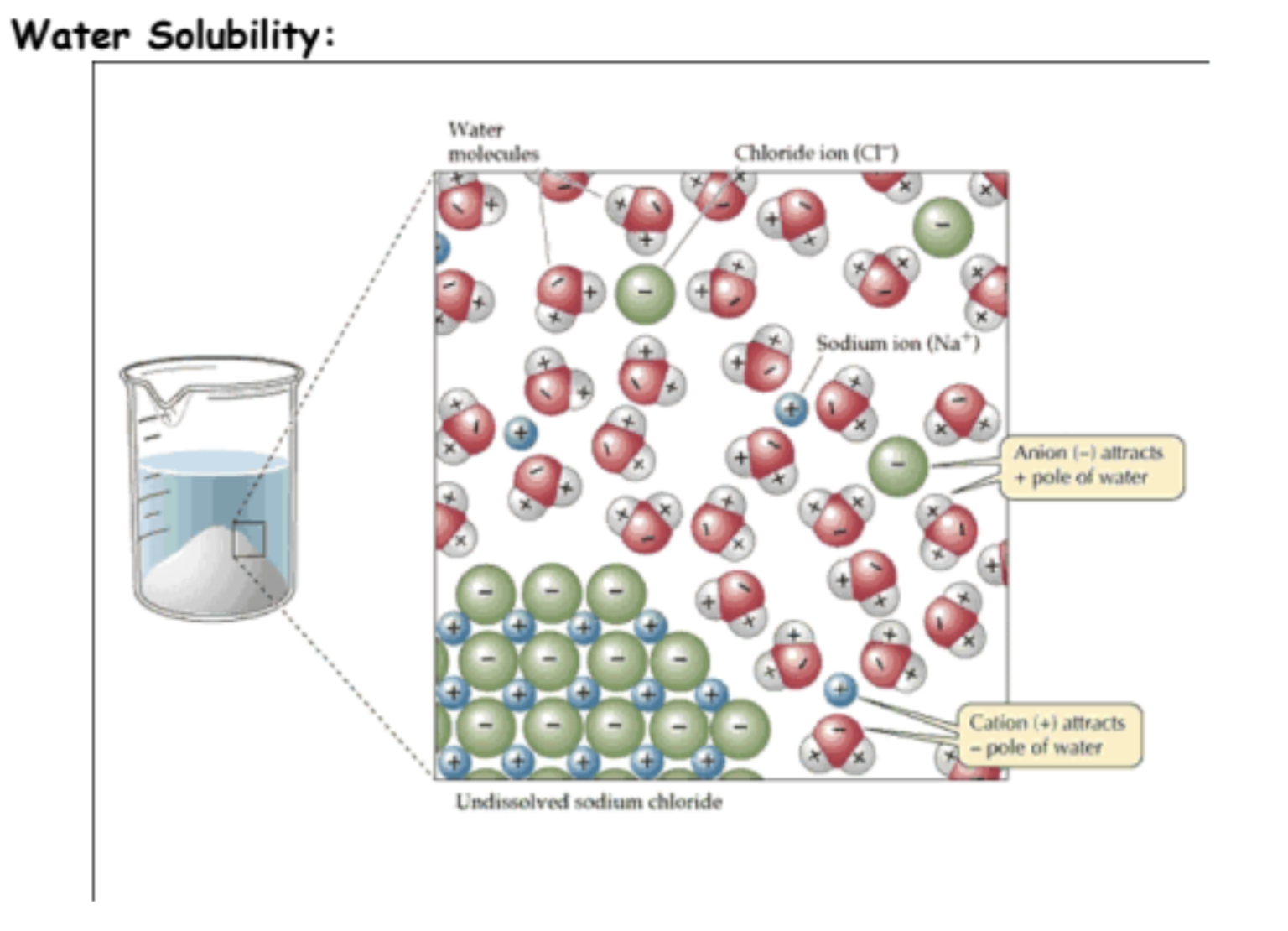
What is Solubility determined by?
determined by how well the particles of one substance will mix with (form attractions with) the particles of another substance. The expression “like dissolves like” is a simple and useful term to remember, and it generally refers to the polarity of substances being mixed.
Lattice Enthalpy
the enthalpy (strength) required to convert 1 mole of an ionic compound into gaseous ions
can be thought of as a direct way of measuring the strength of ionic bonds
stronger ionic bonds lead to higher lattice enthalpies (more energy to break the bonds)
Factors affecting Lattice Enthalpies
More/Higher Charges = Stronger bonds
Smaller Ionic Radii= Stronger bonds
Bond Enthalpies
The energy required to break 1 mole of a bond in the gas phase.
Bond breaking involves the separation of particles which are attracted to each other. It is therefore always an endothermic process.
Factors affecting Bond Enthalpies
More Bonds (Such as Multiple Carbon bonds)= Stronger Bonds
Smaller Atomic Radii= Stronger Bonds
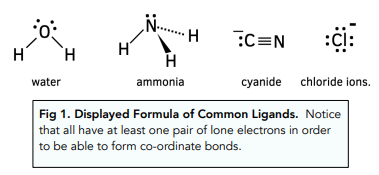
Ligands
species with at least 1 un-bonded electron pair that form coordinate covalent bonds with a metal ion.
Examples of Ligands: (H2O), (NH3), (CO), (CN-), (Cl-), (OH-).
coordinate covalent bonds
a covalent bond (a shared pair of electrons) in which both electrons come from the same atom.
One electron has DONATED its shared electron pair to the other atom to form a covalent bond.
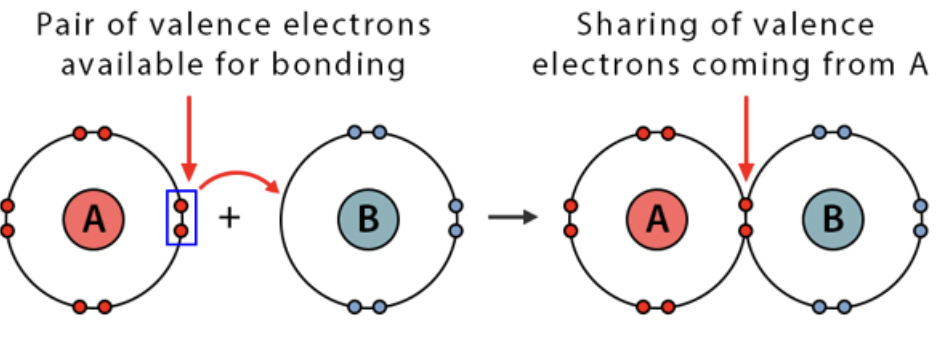
Formation of Transition Metal Complexes
The positive charge density of the metal ions is so great, it attracts the electron pairs of nearby molecules and ions. This results in the formation of a coordinate covalent bond between the metal ion and the ion/molecule (now called a ligand)
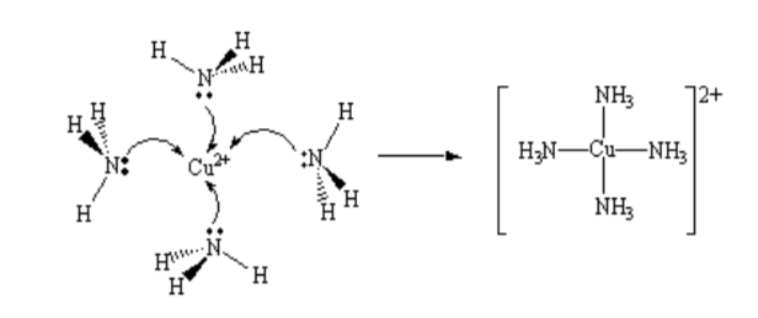
A complex
The large structure formed between the covalently.bonded transition metal ion and ligands. If they have an overall charge, they are called complex ions.
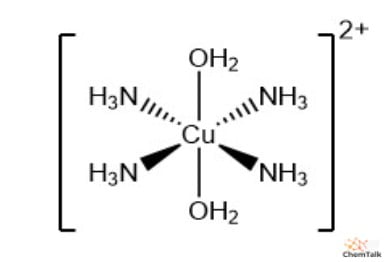
Chemical formulas of complexes & complex ions
Complex ions are usually surrounded by brackets []. The overall charge is written outside the brackets.
e.g:
[Co(NH3)6]3+
[CuCl4]2-
Normal complex:
Fe(H2O)3(CN)3
All Polyatomic Ion Names
+1 CHARGE
Ammonium
-1 CHARGE
Hydroxide
Nitrate
Hydrogen Carbonate
-2 CHARGE
Sulfate
Carbonate
-3 CHARGE
Phosphate
Ionic Hydrates
ionic compound that is linked to at least one molecule of H2O
Water molecules are attracted to the ions through ion-dipole interactions, which fill gaps in the lattice of ions. This makes the ions HYDRATED, where the H2O molecules are considered in their structure/chemical formula.
Hydrous
containing water (in Ionic structures)
Eg: CoCl2•6H2O
Colbat (II) Chloride 6-hydrate
Anhydrous
not containing water (in Ionic structures)
Eg: CoCl2
Colbat (II) Chloride
Formula Units
the term used when referring to the number of particles in an ionic compound, since saying “molecules” is inaccurate.
Example:
1 mole of water= 6.022 × 1023 H2O molecules
1 mole of sodium chloride= 6.022 × 1023 NaCl formula units
Determining # of atoms & # of ions in Ionic compound
For atoms:
MgSO4= 6 atoms
times # of atoms by avogadro’s number
6(6.022 × 1023)= 3.61 × 1024
For ions:
MgSO4= 2 ions
times # of atoms by avogadro’s number
2(6.022 × 1023)= 1.20 × 1024
Empirical formulas of Ionic Hydrates
Determine mass of anhydrous (ionic compound without water) ionic compound and the mass of the water
Determine # of moles in the anhydrous ionic compound and # of moles of water
Divide the number of water moles by the moles of the ionic compound. This gives the number of moles of water per 1 mole of ionic compound, which is the coefficient of water in the chemical formula.
