vid 2 photoelectric effect and particle light
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
blackbody
perfect absorber and emitter
everything at a temperature emits radiation with _ , just shifted to a _ wavelength as things get warmer
same shape curve, shorter
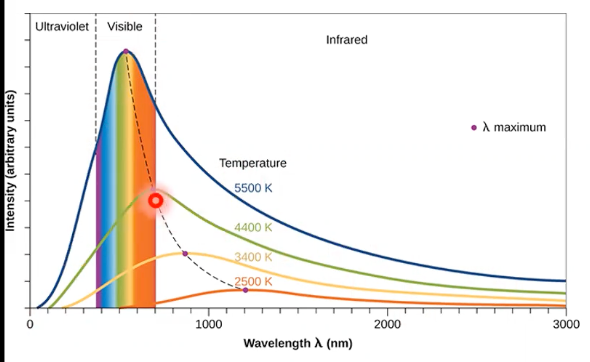
ultraviolet catastrophe
theoretical models showed discrepancies at shorter wavelengths - intensity became infinitely large as wavelength became smaller
planck theorized that
energy assumed certain, quantized values related to frequency
planck’s equation
E = nh(nu)
classical theory of photoelectric effect (wrong)
if frequency increases, so does the number of electrons with no relationship between frequency and KE of electrons
observed photoelectric effect
at lowest frequency (regardless of intensity) no electrons ejected, but as it increases they are, with no increase in number after certain frequency
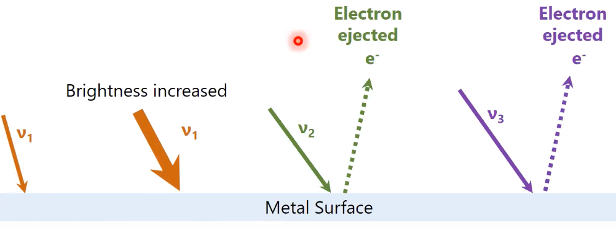
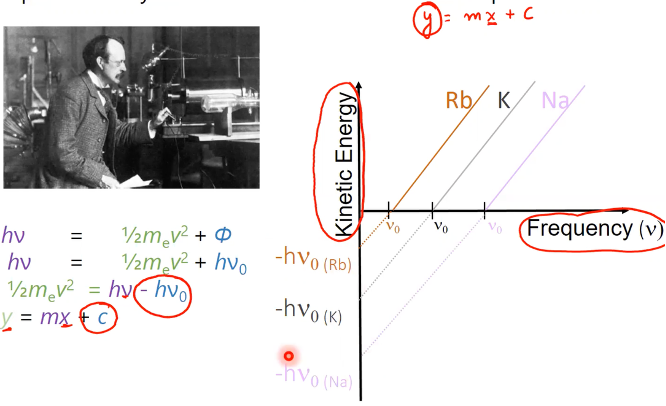
threshold frequency (nu0)
must be reached to eject electrons, differing by metal
what did Einstein say
energy of light is being absorbed by electron and used to eject, with any leftover going to KE
Ephoton = KEelectron + phi
h(nu) = ½ meu2 + h(nu0)