topic 6
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
What is the purpose of converting alcohols into sulfonates?
To make alcohols better electrophiles by replacing OH with a better leaving group (e.g., OTs, OMs, OTf).
what is the general transformation in a nucleophillic substitution at a sp3 carbon?
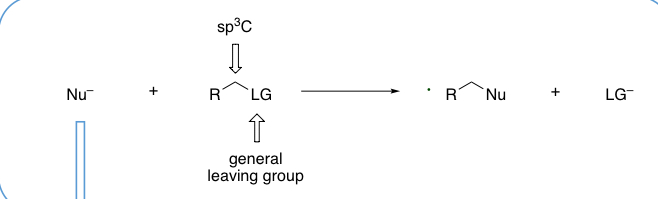
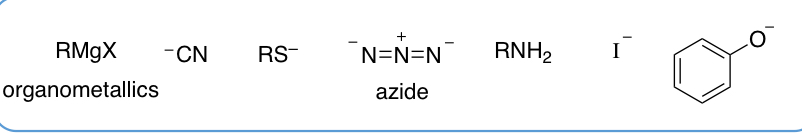
give examples of nucleophiles that can be used in substitution reactions at an sp3 carbon?
How are sulfonates formed from alcohols?
By reacting the alcohol with a sulfonyl chloride (e.g., TsCl, MsCl) in pyridine.
What is the role of pyridine in sulfonate formation?
It acts as both the solvent and acid scavenger (neutralising HCl).
What are some common sulfonate leaving groups?
Tosylate (OTs), mesylate (OMs), triflate (OTf).
How do sulfonates compare to alkyl halides in reactivity?
Tosylates and mesylates are similar to alkyl bromides; triflates are even more reactive than alkyl iodides.
Why are epoxides good electrophiles?
They are strained three-membered rings; ring strain release drives substitution.
How does the polarisation of the C–O bond in epoxides aid reactivity?
The bond is strongly polarised, making carbon more electrophilic.
Why are ethers and regular alcohols poor electrophiles?
Their leaving groups (alkoxides) are poor, and they don’t release ring strain like epoxides.
How can alcohols and ethers be made more reactive electrophiles?
By activation under acidic conditions (Brønsted or Lewis acid), forming better leaving groups like ROH₂⁺.
What is the effect of acid activation on an ether or alcohol?
It protonates the oxygen, creating a better leaving group for substitution.
Why do substitution reactions need multiple mechanisms?
To explain different stereochemical outcomes, e.g., inversion (SN2) vs. racemisation (SN1).
What are SN1 and SN2 reactions?
Two types of nucleophilic substitution mechanisms involving sp3 carbon centres.
What does SN1 stand for?
Substitution Nucleophilic Unimolecular – the rate-determining step involves one molecule (electrophile).
What does SN2 stand for?
Substitution Nucleophilic Bimolecular – the rate-determining step involves two molecules (nucleophile and electrophile).
Draw the SN2 mechanism.
A one-step mechanism where bond formation and bond breaking occur simultaneously with inversion of configuration.
Draw the SN1 mechanism.
A two-step mechanism: (1) loss of leaving group forms carbocation, (2) nucleophilic attack on planar intermediate.
Why does SN2 lead to inversion of configuration?
Nucleophile attacks from the backside of the leaving group, avoiding repulsion and aligning with the σ* orbital.
Why does SN1 lead to racemisation?
The planar carbocation intermediate allows nucleophilic attack from either face, forming a racemic mixture.
Why do 3° alkyl halides undergo only SN1 reactions?
They form stable carbocations, and steric hindrance prevents backside attack required for SN2.
Why do 1° alkyl halides undergo only SN2 reactions?
They cannot form stable carbocations, and have minimal steric hindrance allowing backside attack.
Why can 2° alkyl halides undergo either SN1 or SN2?
They are in between: can form carbocations (less stable), and have some steric hindrance.
How does the rate of an SN2 reaction depend on reactants?
Rate = k[Nu–][R–LG]; depends on both nucleophile and electrophile (bimolecular).
How does the rate of an SN1 reaction depend on reactants?
Rate = k[R–LG]; depends only on the electrophile (unimolecular).
What is the energy profile of an SN2 reaction?
A single transition state with simultaneous bond forming and breaking; no intermediates.
What is the energy profile of an SN1 reaction?
Two-step: high energy transition state to form carbocation, then fast nucleophilic attack.
What is the structure of the SN2 transition state?
Trigonal bipyramidal with partial bonds to nucleophile and leaving group.
What defines an intermediate in a mechanism?
An energy minimum between steps; it can be short-lived but observable.
What defines a transition state?
An energy maximum on the reaction coordinate; cannot be isolated.
Why are epoxides good electrophiles?
High ring strain and polarised C–O bonds promote nucleophilic attack.
Why are alcohols and ethers poor electrophiles?
They have poor leaving groups (RO–), and no ring strain to assist reactivity.
How can alcohols be made better electrophiles?
Convert to sulfonates (tosylates, mesylates, triflates) or activate under acidic conditions.
What role do acids play in substitution of alcohols and ethers?
Protonation improves leaving group ability by forming better leaving species (e.g., ROH₂⁺).
How does solvent affect SN1 and SN2 rates?
SN1: Faster in polar protic solvents; SN2: Faster in polar aprotic solvents.
Why are polar aprotic solvents good for SN2 reactions?
They solvate cations but not anions, leaving the nucleophile more reactive.
Why are polar protic solvents good for SN1 reactions?
They stabilise carbocations and leaving groups through hydrogen bonding.
What does "stereospecific" mean in the context of SN2?
The stereochemistry of the product is directly determined by the reaction pathway (inversion).
Why does SN1 result in loss of stereochemistry?
The planar carbocation allows attack from both sides, scrambling stereochemistry.
How can kinetics confirm a reaction mechanism?
SN2: Rate depends on both reactants; SN1: Rate depends only on electrophile.
What happens to the SN2 rate if both reactants are doubled?
Rate increases 4× (quadratic increase).
What happens to SN1 rate if only nucleophile concentration is changed?
No change in rate; it's zero order in nucleophile.
What is the main factor in determining SN1 vs SN2?
The structure (and substitution) of the electrophilic carbon.
What makes 3° alkyl halides favour SN1?
Carbocation formed is highly stable, making the SN1 pathway lower energy.
What makes 1° alkyl halides favour SN2?
Little steric hindrance, and unstable carbocations make SN1 unfeasible.
What is the stereochemical consequence of SN1?
Racemisation due to equal attack from both faces of the carbocation.
What is the stereochemical consequence of SN2?
Inversion of configuration at the reactive carbon.