Parasitology
1/57
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
define the term parasite
two -species association in which one species (the parasite) lives on or in the second (the host) for a significant period of its life and obtains nourishment from it
always involves two species and the parasite is always the beneficiary
microparasites
can replicate in the host so infection levels can rise rapidly after a single infection - malaria
macroparasites
generally cannot replicate in the host so the levels of infection arendetermined by the number of infection events and number of infective stages
parasite lifecycle that never leaves the host
parasite is never exposed to the external world and completes its development and reproduction in a single host - transmission is by predation /scavenging - rare
eg thricinella spiralis - the spiral threadworm infects host when infected meat is eaten
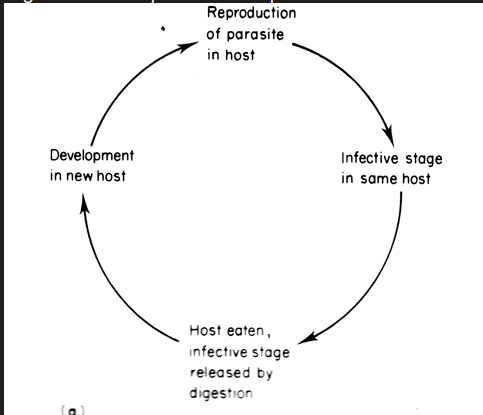
parasite never exposed but development takes place in two or more host species
species in which larval, juvenile or non sexual stages develop is the intermediate host
where one transmits the parasite directly to another ins known as a vector
plasmodium (malaria) , wuchereria (elenphantitis)
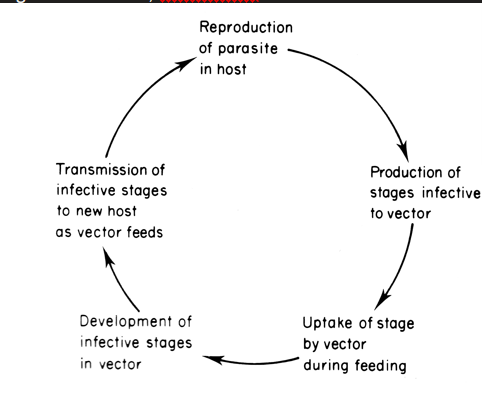
the parasite is exposed to the external world for parts of its lifecycle but not free living
use cycsts or egg shells to protect parasite when it is outside of the host

direct life cycle
direct life cycles when only one host is involved - e.g ascaris
indirect lifecycle
involving intermediate and definitive hosts or different species - eg tapeworms
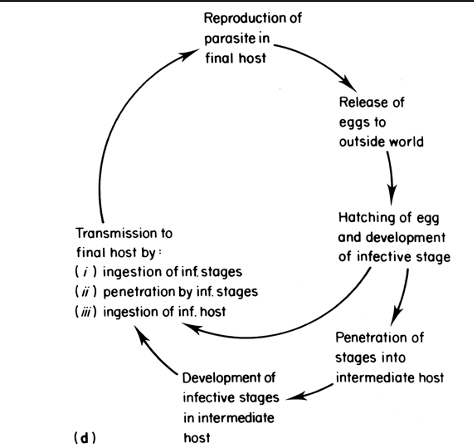
the parasite is exposed to the external world as a free living organism between hosts
reentry to the host can be passive (eating) or by penetration this includes hookworms and schistosomes
role of evolution
evolution acts on the host parasite and on the host parasite relationship
arms race
a series of escalating mutual counter adaptations by 2 lineages to exploit or inhibit exploitation
symbiosis (living together)
a relationship between two dissimilar species living together
commensalism = no metabolic dependence (clown fish and anenomea)
mutualism = obligitory relationship - both benifit
parasitism = one partner benifits a, metabolic dependence may cause harm to the host
if the intimate relationship is onesided then this is parasitic
remarkably common most species have parasites - stralings 126
humans 402
nematodes
range from parasites of plants and animals to free living nematodes
evidence of parasitism from free living and parasitic nematodes
comparing DNA sequences
usually nematodes are only parasitic part of life have some outside life cycle
parasitism evolved at least 7 times in this group
principles of the evolution of parasitism
loss of otherwise essential genes through mutualistic relationship with bacterial endosymbiont
horizontal gene transfer
parasitic feature evolving under free living conditions
Brugia malayi
A parasitic nematode responsible for lymphatic filariasis in humans, transmitted by mosquito bites.
example of a loss of essential genes
20% of gene predictions are B. malayi specific
indicate a huge pool of genes involved in nematode defence
genome shows loss of genes in the nematode encoding for enzymes required for biosynthesis or purine, heme and reboflavin
these vital components are likely sourced from bacteria - wolbachia pr host
pristionchus pacificus
A nematode species known for its role in ecological studies and as a model organism in evolutionary biology, exhibiting both free-living and parasitic lifestyles.
plant parasite. Eggs in root system of host. J2 develops in egg. J2 penetrates plant root; migrates between cells; induces multinuclear cells. Moults x3 to adult.
plant nemotode parasites
of agricultural importance
very different life style to animal parasites
all have a stylet to puncture the plant cell wall to provide muctinucleated plant cells which worms feed on
include root knot nematodes - Meloidogyne incognita and M. hapla
horizontal gene transfer in Meloidogyne incognita and M. hapla
genomes of the two species rich in cell wall degrading carbohydrate- active enzymes (CAZymes (eg cellulases, xylanases)
no counterpart in most ofther animals and is absent from free living nematodes so likely acquired horizontally from plants
parasites and bacteria
for plant nematodes rhizobial bacteria may have been a source of horizontal gene tranfer that enabled the evolution of plant parasitism in nematodes
pre-adaptations
what initiates the evolution from free living life cycles to a complex parasitic one with host switching
are there trends towards parasites and intermediate stages
has parasitism arisin through a series of adatpive steps each one conferring a fitness advantage
the thought is there is some sort of requirment for independent physiological and morphological alterations that enable parasitism
pre adaptations are adaptations to current environments that in the futrue might be coopted to a new function helping the organism in a new niche
ie - an adaptation that conferes fitness in one environment but can be exploited for survival in another
pre-adaptation example
parasite of the large intestine
swallowed during grazing on grass near dung
little diferent between dung ans colon contents
pre-adaptation - cuticle, tolerates toxic enzymes and anaerobic conditions
would allow for the evolution of parasitism
but pre-adaptation remains hypothetical
nematode associations with insects
may represent pre adaptations
tolerance to toxic enzymes
adapt to low oxygen conditions
form dauer larvae
phoretic associations
necromenic association
not yet parasitic but could develop metabolic dependence on its host
dauer larvae stage
these are specialised, arrested non feeding stage - specialised for suvival and dispersal
phorecy- the use of insects for transportation (not species specific)
necromy - dauer associates with insects then waits for its death to feed on the microbes that grow on its carcass
is the dauer stage preadaptation?
both the dauer stage and parasitic larvae have speciealised cutical
both cannot feed and resume development in a later life cycle
molecular similarities - a conserved endocrine singalling mechanism involving dafachronic acid controls formation of dauer and infective larvae
caenorhabtitis elagans
L2 haas to sense the environment to decide wether to grow or arrest
dauer has to sense environment (eg temp) to decide when to resume developments
C. elegans – development of dauer is default developmental behaviour.
Specified when DAF-12 is not occupied by ligand
Under inducing conditions- generate DA -binds to DAF-12 to specify non dauer larvae
strongyloides papillosus
has adult parasites and free living parasitic lifestyle
halfway house between free living and parasite
it has a free living developmental stage then a choice between a infective larvae and a non infective larvae
Add DA to S. papillosus larvae passed from the host as well as the progeny of the free-living adults both resulted in the development of non-infective larvae suggesting that DA–DAF-12 signalling acts at the two places indicated.
anclyostoma (hookworm)
when the development of the parasitic stage is constitiutive not faculative can DA still turn it off
The role (if any) of DA in the development of life cycles such as these, remains to be investigated.
can DA be used to control nematode transmission?
pre-adaptation example
evidence from pristionchus pasificus
has a necromenic association with scarab beetles is this a intermediate between free living nematodes and parasites
it has a increase in detoxifying enzymes seen in lots of parasites
but it also has glycosyl hydrolase encoding genes in its genome - not found in any other non-parasitic nematode
malaria
found in areas above 15 degrees as the mosquito vectors require this higher temp
plasmodium
species resonsible for causing malaria
>100 species
4 are transmissible to humans
P. falciparum - most wide spread
P. vivax - worldwide - tropics
P. ovale - west africa
P. malariae - worlldwide but patchy
needs temps of over 15 degrees and cant survive above 3000 m
life cycle - simple
4 phases - 1 sexual and 3 asecual phases with multiplication
injected into humans by a feeding female mosquito
sporozoite is injected into humans and it turns into merozoites as it moves into the liver
leaves the liver and moves into red blood cells so it can be taken up by the mosquito
another replication stage and each merozoite turns into 30-40 more
some turn into the sexual form and turn into gametocytes
burst out of red blood cells and invade others in a synchronous event
this mass bursting of red blood cells and release of waste is what produces the symptoms
when fed on by another mosquito they are sucked up with the red blood cell
digestion of the red blood cells in the mid gut of the fly triggeres the gametocytes to turn into zygotes and become sporozoites again
phases of the life cycle
1 - sexual phase occurs only in the mosquitoes
the second asexual phase is in the liver
third asexual pahas in blood repeated many times
every phase begins with feeding and growth
every phase ends when new invasive parasites appear
in the the third phase some parasites become sex cells the gametocytes
start a new cycle if taken up by anopheles mosquitos
test for infection
take a pinprick of the subject, stain it and look under the microscope
shizogony
a shizont = mass of parasite in red blood cells
shizogony = bursting out of the red cell
red blood phase starts to deform and look diferent
the sexual phase
the mosquito is the definitive host
ingested gametophytes swell
discharge osmophillic bodies in RBC
disrupts RBC membrane and releases gametophytes
male gametophyte DNA is replicated 3 times resulting in 8 sets of DNA
8 kinetosomes formed in microtubular organising
kinetosome is the base for flagellum and 8 flagella are formed
gamete explodes releasing flagellum
called exflagellation = release of spermatozoa
these then actively swim to female gametocytes and fertilize them
forms a zygote that in the next 5-10 hours develops into an ookinete
major changes occur and forms apical complexes
ookinent penatrates the mosquito midgut wall, transforms into an oocyst. this is the first asexual phase
first asexual phase
oocysts growsto 80 um in diameter
oocyst prjects into haemocoel of insect and feeds on teh heamoglobin of the blood meal
DNA replication then occurs
each oocyst contains at least 1000 sporozoites
burst out of sporocyst and migrate to mosguitos salivary gland
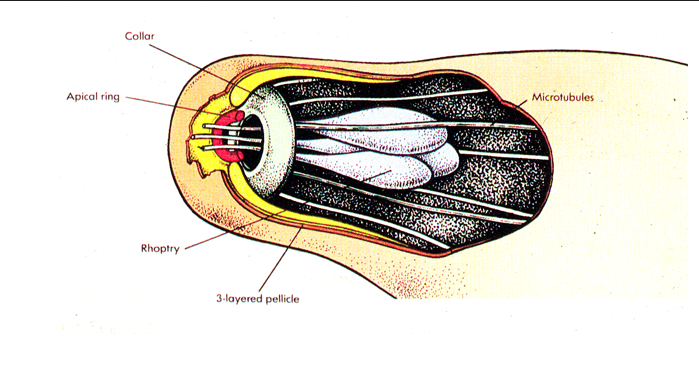
apical complexes
plasmodium is a single celled organism but has a front and back with a apical complex to invade cells
ookinete uses apical complex to digest epithilium of gut wall
second asexual phase
within 30-40 minutes after a mosquito bite they end up in your liver cells and go into macrophyte like cells called kupffer cells
then leave kupffer cells go into hepatocytes then go through 2-3 more kupffere cells before they stop
hepatocytes are nutrient rich
rapid growth = loss in sporozoite morphology rounds up and becomes a trophozoite
divides into 10-30,000 merozoites
at this point infected person shows no symptoms
i P. viavax there is a latent stage called hypnozoites that trophozoites can become and cause relapses years later
the parasite in cells
parasite moves into parasitophorus vacuole - is not free in the cytoplasm
the third asexual phase
in red blood cells
merozoites burst out of heptacytes and invade RBCs
once inside they ingest haemoglobin
2-3 days to grow and syncronously burst out in schizogony
after several blood cycle some become gametophytes and remain dormant in RBC until bitten by mosquito
how does the parasite know what cells to invade
receptor recogition and binding
erthrocyte deformation
red blood cell invasion
RBC is normally very rigid - not phagocytic
this is due to its cytoskeleton network
highly ordered and hard to disrupt
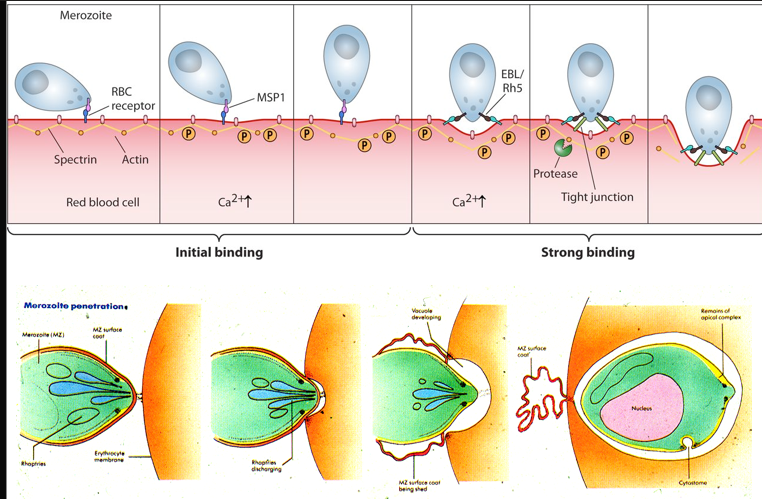
parasites attach to red blood cell and roll around on the RBC surface for a while
entry following the alignment of apical end
AMA-1 and RON-2 secreted by the parasite - secretes its own receptor which binds to RBC
in P falciparum PfRh5 from the parasite binds to basigin (blood group molecule) - essential for RBC invasion
attachment of fibrils to parasite surface coat
tight junction is formed subsequent invagination then the surface coat of the parasite is sloughed of and not engulfed in the parasitic vacuole
invasion
requires dramatic re-organisation of the cytoskeleton of both parasite and host cell
to invade it needs only form
rhopteries to form tight junctions and penetrate the RBC membrane.
micronemes and secretory organelles that play a crucial role in parasite entry.
post entry of malaria
the development of feeding stages
trophozoites ptoduce enzymes to degrade haemoglobin
novel transporter system - maurer’s clefts
then you result in deformed shape of the RBC
RBC becomes deformed with lumps on its surface (knobs)
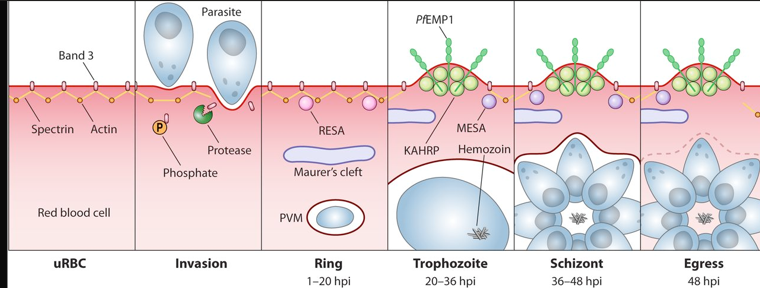
importance of knobs
Knobs are protrusions on the surface of infected red blood cells that facilitate adherence to blood vessel walls, enhancing parasite retention in the circulation and contributing to severe disease symptoms in malaria.
this keeps the parasite away from the spleen which controls malarial infections
can cause damage to brain and kidney esp P flaciparum
fatal consequences of cerebral malaria
toxoplasma gondii
single celled protozoa
known as the king of parasites as it can infect almost any warm blooded animal (birds and mammals)
the apicomplexan family
of parasites, including Plasmodium and Toxoplasma, characterized by an apical complex used for host cell invasion.
obligate intracellular protozoan parasites
affects 1/3 of humans
infects almost anything warm blooded
three strains of toxoplasma
members of the cat family are the only definitve host
history
1908 discovered in the gundi (rodent from middle east)
1939 - congenital infection in a child
1960 carnivorism (lab hypothesis)
1965 (carnivorism human)
1970 - found in cat faeces
1970 - life cycle found out by bill hutchison
Demonstrated that toxoplasma was a parasite of cats that shed oocystes in the faeces
lifecycle
felines ingest tissue infected with bradyzoite cystes (or oocystes from another feline with the sporozoites)
the cysts burst and bradyzoites invade the intestinal epithelium (as do sporozoites)
both forms can differentiate into male and female gametes and after fertilisation become oocysts
these oocytes contain sporozoites and are shed from the feline - several million a day for up to 3 weeks
if any warm blooded animal ingests thes the sporozoites will be released into the intestine where they invade it and diferentiate into tachyzoites
disseminate arount the body and become bradyzoite cysts again
if a cat eats these cysts the cyle begins
problems of toxoplasma
can form brachyzoites in the brain or in the fetus of pregnant women
can cause abourtion or defects
if you are immunosupressed the toxoplamosis can reactivate and migrate to the brain
stages of infection - tachyzoites
intermediate hosts
rapid intracellular growth and accumulation
targets almost any nucleated cell
secreted into the blood stream
results in acut disease (parasitemia)
limited by immune response and transfomation into cyst-forming bradyzoites
is probably that the host trying to control the tachyzoites results in transformation into bradyzoite cysts - particularly TH1 response
immune response keeps infection in check and dormant
toxo secretes molecules inhancing th1 response - cyclophillin 18
life cycle - bradyzoites
intermediate hosts
slow multiplication rate
forms cysts in neural and muscular tissue
persist and cause chronic disease
if immunocompromised they can cause acute encephalitis
life cycle - sexual stage
in definitive host (feline)
in intestine oocysts form in the endotheluim
unsporulated oocysts shed in faeces - 3-18d)
sporulate in 3 week period
contaminate water, soil, fruits and vegetables
very stable in warm and humid environments
why is it only feline hosts
linolec acid is required for sexual reproduction of toxoplasma
felines are the only mammals that lack delta-6-desturase in their small intestine - enzyme that breaks down linoleic acid
thus cats have an excess of linoelic acid in their intestine allowing toxoplama to develop sexually
thus experimentally you can increase the levels of linoleic acid in other species and they become the definitie host
allows for easier study with mice as cats are not great in the lab
parasite movement
it glides
use genomic info to study hteir biology
a lot harder to do this in malaria parasites
movement relies on an intricate linear motor sytem which is sandwiched between the parasites plasma membrane and pair of membranes known as the inner membrane complex
apical complec - rhotperies and microneme deliver molecules to initiate cell invasion
actin and myosin together with special gliding associated proteins are involved
effects of toxoplasma
women are seropositive for toxomplasma have more sons than uninfected women
mice infected produce more males in litters
maybe this is as males are known to roam more than females = more likely to be eaten
treatment
many many people have this
treated with
sulfonamides and pyrimethanime
spiramycin to reduce congenital transmissions
live vaccine of cystless strain available for sheep
no human vaccine
no drugs target encysted bradycytes
leishmania
incidence of 400,000 a year and a total 12 cases in the world
20,000 death/year in india
many diferent species
transmitted by sandfly vectors - major groups
phlebotomus
lutzomyia
obligate intracellular parsites
live in macrophytes
the forms infecting macrophages are very similar reult in diverese clinical manifestations
found as far north as the mediteranean basin
lifecycle
live in sandfly which are much smaller than mosquitors so the females saw into the skin to eat and deliver anticoagulant and parasite with it
initially the parasite has a glagellum (promastagotes) then lose it when they enter macrophages (amastigotes)
grow by binary fission in the macrophage then burst out
taken up by sand flies when feeding and become metacyclic form (ready to infect humans again