17. magnetic properties of substances
1/6
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
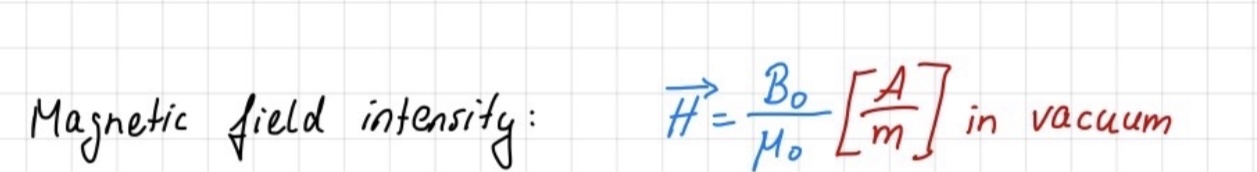
magnetic field intensity
Magnetic field intensity is represented by lines around a magnet, with outward lines from north poles indicating magnetic flux. The total magnetic field passing through a surface is termed magnetic flux.
Magnetic flux density (B) is defined as the magnetic flux (Ф) divided by the surface area (S) perpendicular to the magnetic field. It's measured in Tesla (T).
magnetic permeability
Magnetic permeability refers to a material's ability to concentrate magnetic lines of flux. Materials with high permeability are easily magnetized.
Relative permeability (ur) is the ratio of a material's permeability (u) to the permeability of a vacuum (40).

dia -, para - , ferromagnets
Diamagnetic materials: These materials have no permanent magnetic dipole moment and are weakly repelled by both poles of a magnet. When exposed to a magnetic field, diamagnetic materials develop a magnetic moment opposite to the applied field. Examples include water, copper, and gold.
Paramagnetic materials: These materials have unpaired electrons, which align with an external magnetic field, resulting in a weak attraction to the field.
However, paramagnetic materials lose their magnetization when the external field is removed. Examples include aluminum, platinum, and oxygen.
Ferromagnetic materials: These materials have strong permanent magnetic dipole moments due to the alignment of atomic magnetic moments within domains. When exposed to a magnetic field, ferromagnetic materials become strongly magnetized in the direction of the field. They retain their magnetization even after the external field is removed. Examples include iron, nickel, and cobalt.
magnetic hysteresis
Magnetic hysteresis occurs in ferromagnetic materials like iron, nickel, or cobalt when subjected to changing magnetic fields. This results in energy loss as heat.
These materials consist of small magnets that align with applied fields but resist changing direction, making them useful for applications like transformers.
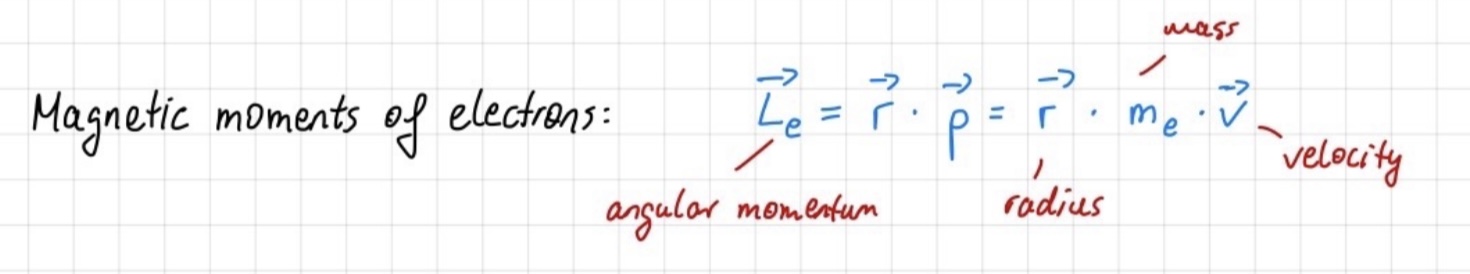
mechanical and magnetic moments of electrons and atoms
Mechanical Moment of Electrons: This refers to the angular momentum of an electron as it orbits the nucleus of an atom. According to quantum mechanics, electrons can only occupy certain discrete energy levels, each associated with a specific angular momentum. The mechanical moment of electrons contributes to properties such as an atom's magnetic behavior and its ability to interact with electromagnetic fields.
Magnetic Moment of Atoms: Atoms possess an intrinsic magnetic moment due to the magnetic moments of their constituent particles, including electrons and the nucleus. The magnetic moment arises from the spinning motion of charged particles (electrons) and the intrinsic magnetic properties of particles like protons and neutrons. The overall magnetic moment of an atom is the vector sum of the individual magnetic moments of its constituent particles.
Electron paramagnetic resonance
Electron paramagnetic resonance (EPR), also known as electron spin resonance
(ESR), is a spectroscopic technique used to study materials with unpaired electrons, such as free radicals, transition metal complexes, and some defects in crystals.
In EPR, the material is placed in a strong, constant magnetic field. When exposed to weak radio frequency radiation, electrons in the material absorb energy and undergo transitions between different energy levels. This absorption results in a characteristic EPR spectrum, which provides information about the local environment and magnetic properties of the electrons in the material.
magnetic nanoparticles for diagnostics and therapy
Diagnostics: In diagnostics, magnetic nanoparticles are used as contrast agents in magnetic resonance imaging (MRI). These nanoparticles can be functionalized with targeting ligands to specifically bind to biological targets such as tumors or diseased tissues. When introduced into the body, they enhance the contrast in MRI images, allowing for more accurate visualization and diagnosis of diseases.
Additionally, magnetic nanoparticles can be used in magnetic particle imaging (MPI), a promising imaging modality that provides high sensitivity and spatial resolution.
Therapy: In therapy, magnetic nanoparticles can be used for targeted drug delivery and hyperthermia treatment. By attaching therapeutic agents such as drugs or genes to the surface of magnetic nanoparticles and guiding them to specific targets using external magnetic fields, it's possible to achieve localized and controlled drug delivery. Furthermore, magnetic nanoparticles can generate heat when exposed to an alternating magnetic field, a phenomenon known as magnetic hyperthermia. This heat can be used to selectively destroy cancer cells while sparing healthy tissue, offering a potential non-invasive treatment option for cancer therapy.