Endothermic / Exothermic reactions and Enthalpy
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
What is Enthalpy change ?
a measure of the amount of energy absorbed or released during chemical reactions.
given the symbol ∆H
determined by subtracting the enthalpy of the reactants from the enthalpy of the products.
AKA HEAT REACTION (HEAT USED ETC)
What is enthalpy?
stored chemical energy of a substance
All chemicals have a certain amount of stored chemical energy, or enthalpy.
the change in enthalpy that occurs in a chemical reaction that is most important to consider.
How is enthalpy change calculated?
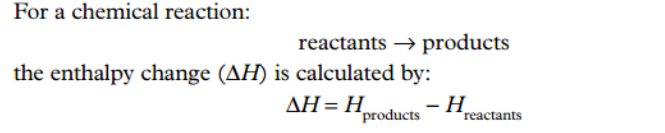
What is an energy profile diagram?
represents the energy changes that occur during the course of a reaction.
describe enthalpy change in a exothermic reaction
When enthalpy of the products is less than the enthalpy of the reactants, energy is released from the system into the surroundings
The system has lost energy, so ∆H has a negative value.
combustion reactions (which are exothermic reactions), ∆H < 0
describe enthalpy change in a endothermic reaction
total enthalpy of the products is greater than the total energy of the reactants, energy must be absorbed from the surroundings, so the reaction is endothermic.
The system has gained energy, so ∆H has a positive value, i.e. ∆H > 0
How is Enthalpy represented in a thermochemical equation?
∆H value in a thermochemical equation usually has the units kJ mol-1 (kilojoules per mole).
amount of energy (in kJ) signified by the ∆H value corresponds to the mole amounts specified by the coefficients in the equation.
How is Enthalpy represented in a thermochemical equation (explain the exotheric respiration example)?
CH12O(aq) +60, (g) 6CO(g) + 6H2O) AH=-2803 kJ mol-1
when 1 mole of glucose reacts with 6 moles of oxygen to produce 6 moles of carbon dioxide and 6 moles of water, 2803 kJ of energy is released to the surroundings.
with enthalpy written instead:
CH12O(aq) +60,(g) → 6CO2(g) + 6H2O(l) + 2803 kJ mol1
still equal to -2803 kJ mol1.
energy is written on the right-hand side of the equation as a 'product' in this exothermic reaction.
What is an exothermic reaction?
chemical reaction in which energy is released
total chemical energy of the products of the chemical reaction is less than the total chemical energy of the reactants.
This 'lost' energy is released to the surroundings (heat).
Energy 'exits' the reaction system
where do you write energy in a exothermic thermochemical reaction?
The released energy can be shown in a chemical equation by writing 'energy' on the product side of the arrow.
What is an endothermic reaction?
total chemical energy of the products is greater than the total energy of the reactants, energy will be absorbed from the surroundings.
Energy 'enters' the reaction system
energy that is required can be written on the reactant side of the equation arrow.
what happens when an endothermic reaction happens in a container?
container may feel cold to the touch.
the reaction system is absorbing heat from the surroundings, leaving the environment cooler.
difference between systems and surroundings?
system: collective substances in the reaction such as the reactants and products. surroundings: everything around the reaction such as the reaction flask and the room.
differnce between endo and exo?
If the total chemical energy of the products is less than the total energy of the reactants, energy will be released from the system into the surroundings. This is called an exothermic reaction.
If the total chemical energy of the products is greater than the total energy of the reactants, energy will be absorbed from the surroundings. This is called an endothermic reaction.
for further calculation explanations
refer to ch 10.2 in pearson pg 216 onwards alos read + attempt w.e in ch 11.2 and 11.3 for more