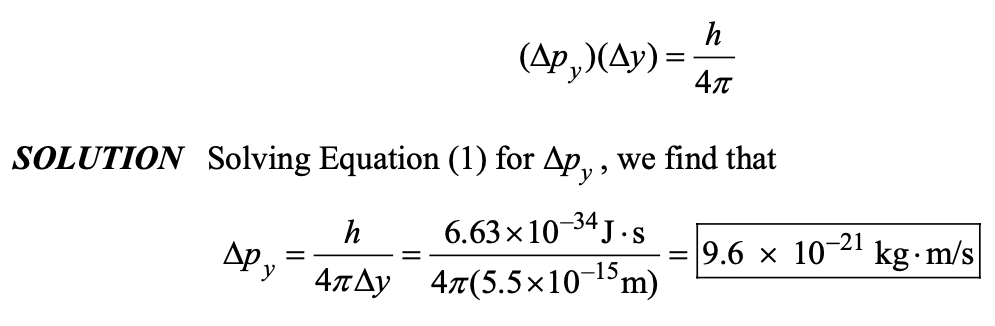Ch. 29 - Particles & Waves
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
Continuum - Black body radiation
Radiation emitted by a heated object
Examples of heated objects that emit radiation:
Light bulbs
Hot coals
Ice cubes
Starlight and sunlight can be…
Approximated as a black body radiation
Emission spectra is measured using:
A spectroscope
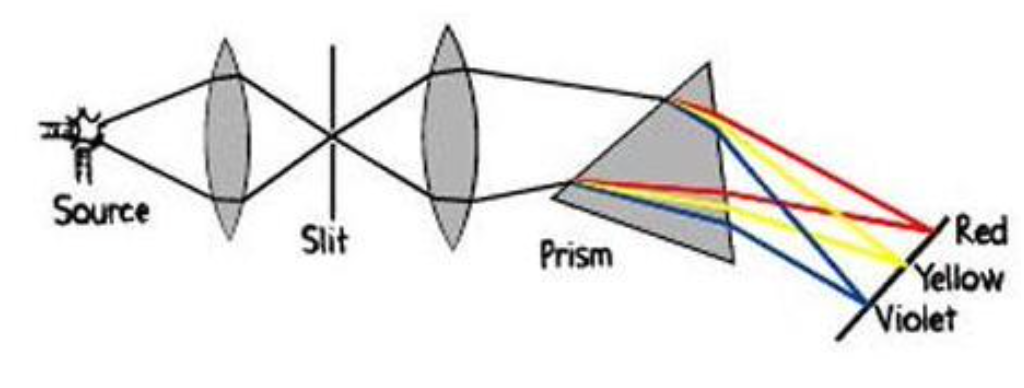
Emission spectra
The characteristic pattern of light frequencies emitted by elements is determined by their electron energy levels
Gasses and vapors in emission spectra
Discrete spectrum (single lines on a black background)
Every element has its own characteristic pattern of electron energy levels and therefore…
Emits light with its own characteristic pattern of frequencies, emission spectrum, when excited.
Absorption spectra
Dark lines agianst a rainbow-colored background.
Absorption is opposite of emission
Electrons absorb specific wavelengths to raise up to a higher energy level
Examples of absorption spectra
Stellar atmospheres
Cool interstellar clouds
Hydrogen series
Emission spectra of hydrogen
Equation determined by Johann Balmer (1855)
Line spectrum equations
Lyman series
Balmer series
Paschen series
Modern physics =
Quantum physics
Blackbody radiation
An object that absorbs all incident radiation, then reemits it according to its temperature (ex. the sun). A
All bodies, no matter how hot or cold,…
Continuously radiate electomagnetic waves.
Is light a wave or colored particles?
Three theories:
Corpuscular theory of light
Wave theory of light
Absorption and emission of light
Corpuscular theory of light
Aristotle and Newton
Wave theory of light
Huygens and Maxwell
Absorption and emission of light
Plank and Einstein - Fathers of quantum theory
Einstein’s nobel prize
Only certian wavelengths eject electrons - The Photo-electron Effect
LIGHT - comes in “packets” of energy, called quanta
Now known as PHOTONS
Photoelectric Effect
Light is capable of ejecting electrons from various metal surfaces
The photoelectric effect depends on…
The light frequency and intensity only above the cut-off frequency.
Photoelectric effect phenomenon
Experimental evidence that light consists of photons
When light shines on a metal…
A photon can give up its energy to an electron in that metal.
Work Function
The minimum energy required to remove the least strongly held electrons
Photon’s energy
E = hf
Work function equation
KE = hf - Wo
fo = Wo/h
The Photoelectric Effect for a Silver Surface Example
The work function for a silver surface is 4.73 eV. Find the minimum
frequency that light must have to eject electrons from the surface.
263 nm (UV)
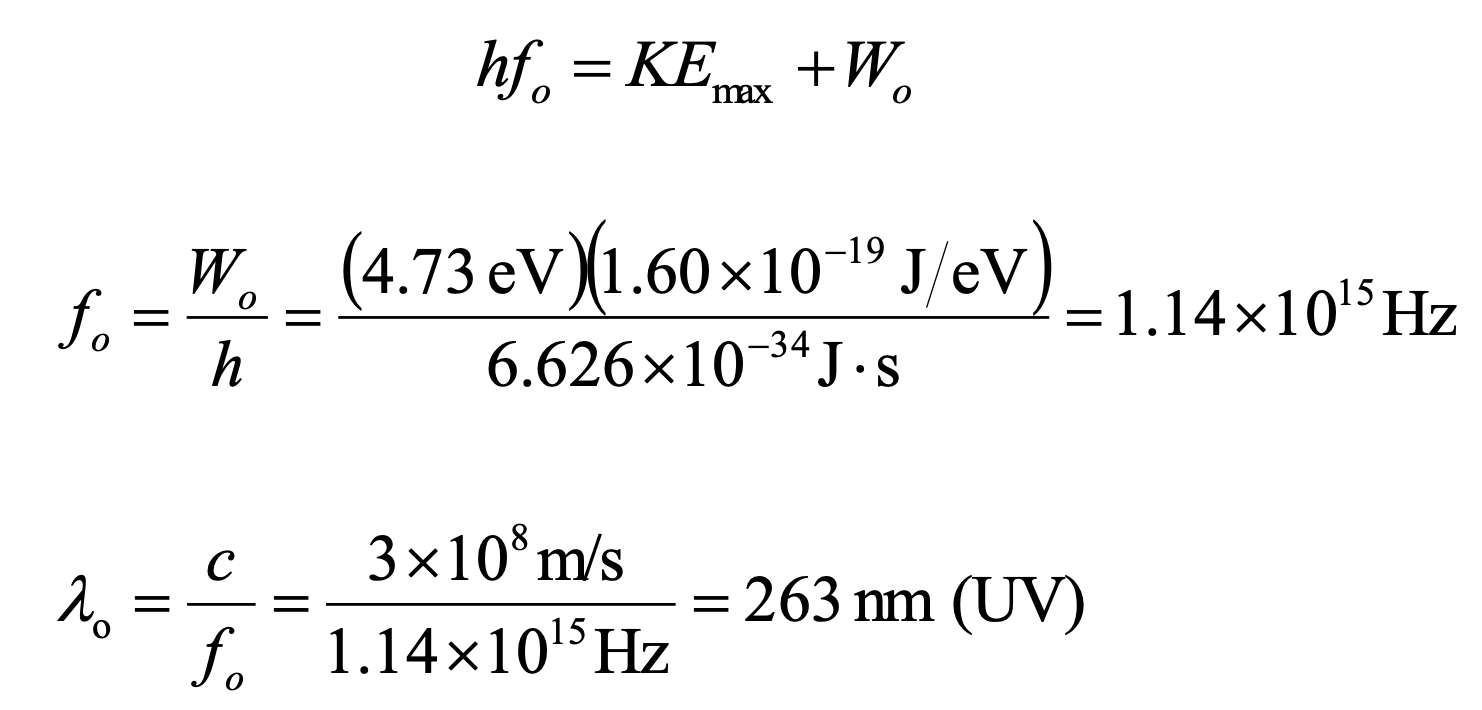
The Photoelectric Effect for a Silver Surface Example (Cont.)
UV radiation with a wavelength of 95 nm impinges on a silver surface
whose work function is 4.73 eV.
a) Will this light eject electrons from the surface?
b) What would be the speed of the ejected electrons?
a) Yes, the light will eject electrons from the surface
b) 1.72 × 106 m/s

Compton Effect
The scattered photon and the recoil electron depart the collision in different directions. The scattered photon must have a smaller frequency.
Due to conservation of energy…
Scattered photon must have a smaller frequency (compton effect).
Incident X-ray photon’s energy =
Scattered photon’s energy + KE recoil electron
hf = hf’ + KE
Momentum of incident photon =
Momentum of scattered photon + momentum of recoil electron
Solar Sails and the Propulsion of Spaceships Example
One propulsion method that is currently being studied for interstellar
travel uses a large sail. The intent is that sunlight striking the sail
creates a force that pushes the ship away from the sun, much as wind
propels a sailboat. Does such a design have any hope of working and,
if so, should the surface facing the sun be shiny like a mirror or black,
in order to produce the greatest force?
Yes, it can work.
For best results the surface facing the sun should be shiny like a mirror.
Photons
All electromagnetic radiation consistes of particle-like entities called…
Photons have:
Energy and Momentum
Energy equation
E = hf
Momentum equation
p = h/λ
p = hf/c = E/c
Wave particle duality - Light
Light interferes with itself when viewed thorugh 2 slits
Wave particle duality - Electrons
2-slit experiment for electrons shows particle behavior
When a beam of electrons is used in a double slit experiment, a fringe pattern occurs, indicating interference effects.
Waves can exhibit…
Particle-like characteristics.
Particles can exhibit…
Wave-like characteristics.
de Broglie wavelength equation
λ = h/p
The de Broglie Wavelength of an Electron and a Baseball Example
Determine the de Broglie wavelength of (a) an electron moving at a speed
of 6.0x106 m/s and (b) a baseball (mass = 0.15 kg) moving at a speed
of 13 m/s.
a) 1.2 × 10-10 m
b) 3.3 × 10-34 m
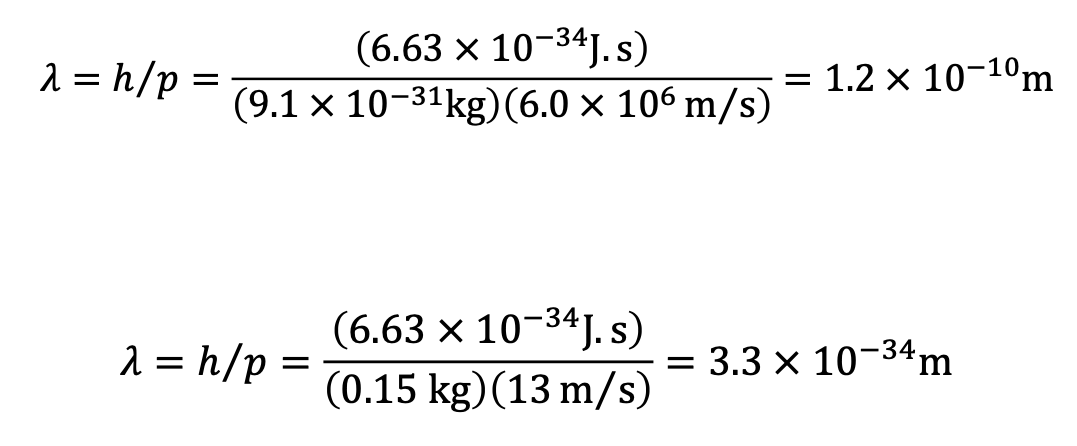
Example Question:
Two particles, A and B, have the same mass, but particle A has a
charge of +q and B has a charge of +2q. The particles are
accelerated from rest through the same potential difference. Which
one has the longer de Broglie wavelength at the end of the
acceleration?
(a) Particle A, because it has the greater momentum, and, hence,
the longer de Broglie wavelength.
(b) Particle B, because it has the greater momentum, and, hence,
the longer de Broglie wavelength.
(c) Particle A, because it has the smaller momentum, and, hence,
the longer de Broglie wavelength.
(d) Particle B, because it has the smaller momentum, and, hence,
the longer de Broglie wavelength.
(e) Both particles have the same de Broglie wavelength.
( c ) Particle A, because it has the smaller momentum, and, hence, the longer
de Broglie wavelength.
Neuron diffraction
A manifestation of the wave-like properties of particles
The Heisenberg uncertainty principle
We can know exactly posistion, but not momentum, or we can know mementum exactly, but not position.
Never both with accuracy.
Also true for time and energy.
Heisenberg uncertainty equation
(ΔE)(Δt) > or = h/4π
E = Uncertainty in the energy of a particle when the particle is in a certain state
t = time interval during which the particle is in that state
What if Planck’s Constant Were Large? Example
A bullet leaving the barrel of a gun is analogous to an electron passing
through the single slit. With this analogy in mind, what would hunting
be like if Planck’s constant has a relatively large value?
Hunting would be nearly impossible using conventional firearms. Bullets would behave like quantum particles, exhibiting unpredictable, wave-like behavior.
Heisenberg uncertainty principle Example
Suppose that the momentum of an electron is measured with complete accuracy (i.e., the uncertainty in its momentum is zero). The uncertainty in a simultaneous measurement of the electron’s position __________.
(a) is also zero
(b) is infinitely large
(c) is some finite value between zero and infinity
(d) cannot be measured, because one cannot measure the position and momentum of a particle, such as an electron, simultaneously.
(a) is also zero
Heisenberg Uncertainty Principle Example 2
A proton is confined to a nucleus that has a diameter of 5.5 10−15 m. If this distance is considered to be the uncertainty in the position of the proton, what is the minimum uncertainty in its momentum?
9.6 × 10-21 kg . m/s