Chapter 16: Conjugated Pi Systems and Pericyclic Reactions
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
3 Classes of Dienes
Cumulated (adj. = bonds)
Conjugated
Isolated
Diene
2 = bonds b/t C atoms
Conjugated dienes can be prepared from allylic halides via an elimination process, Potassium tert-butoxide is used because:
t-BuOK is sterically hindered only acts as a base
Why is the single bond shorter in the conjugated diene? The C2-C3 bond in 1,3 butadiene is formed by the _ of 2 _ hybrid orbitals.
overlap
sp2
Since sp2 orbitals have more s character than sp3 orbitals, the electron density is _ to the nucleus in an sp2 orbital, so the bond is shorter.
closer
Stability of Conjugated Dienes?
tetra sub > tri sub > TRANS di sub > CIS di sub > MONO cub
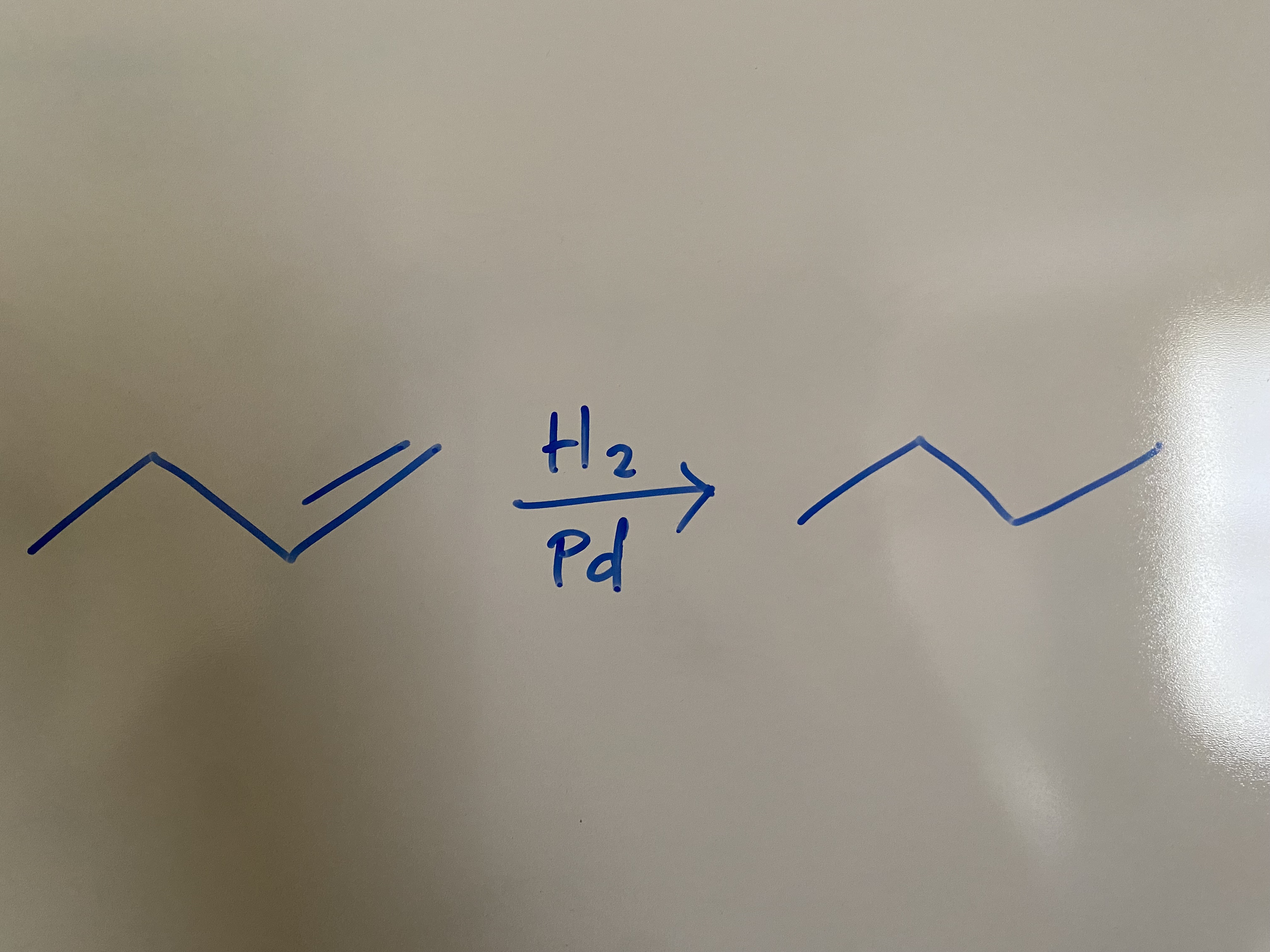
-126 kJ/mol
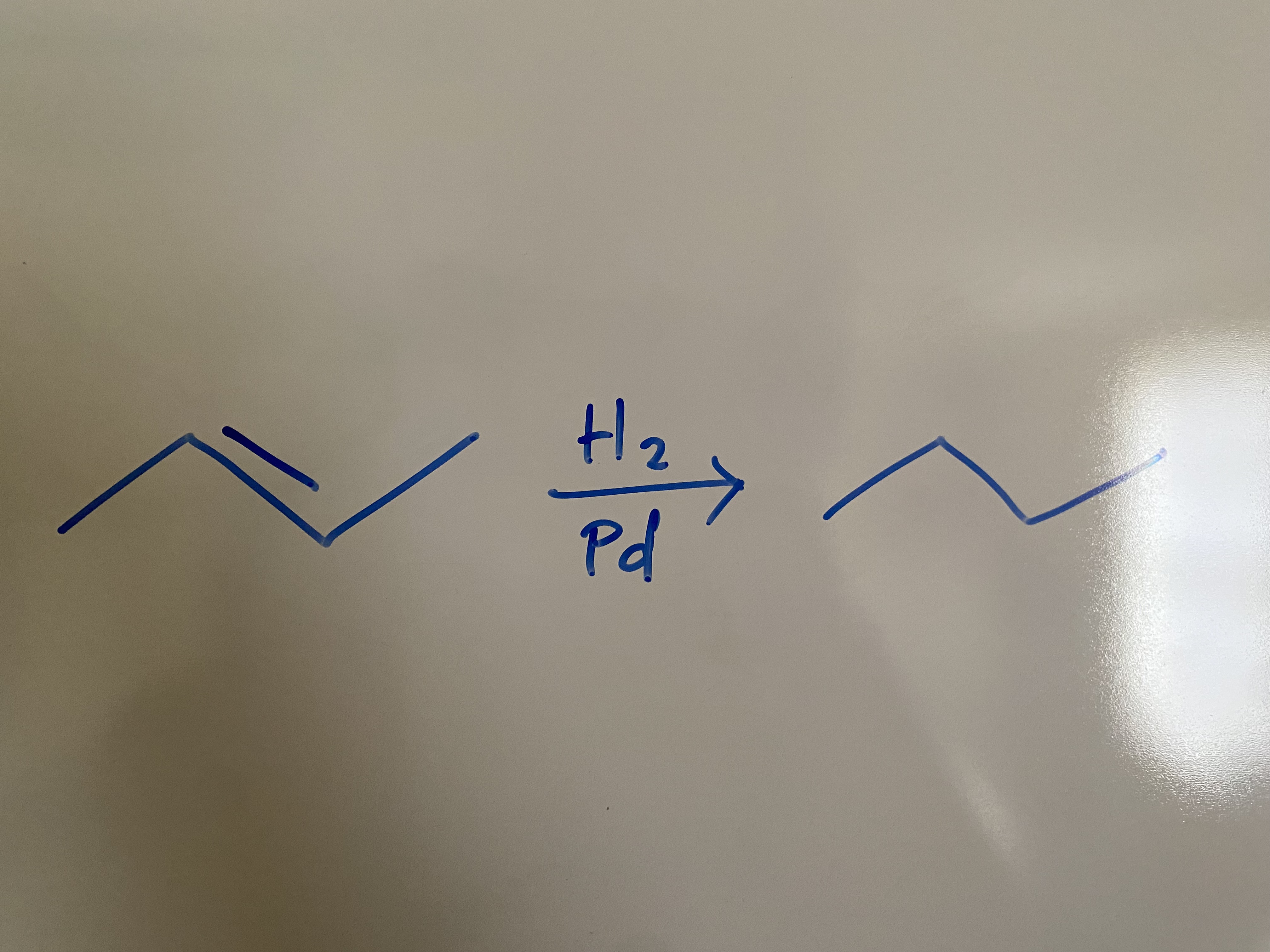
-116 kJ/mol
The _ the heat of hydrogenation, the more stable the double bond.
lower
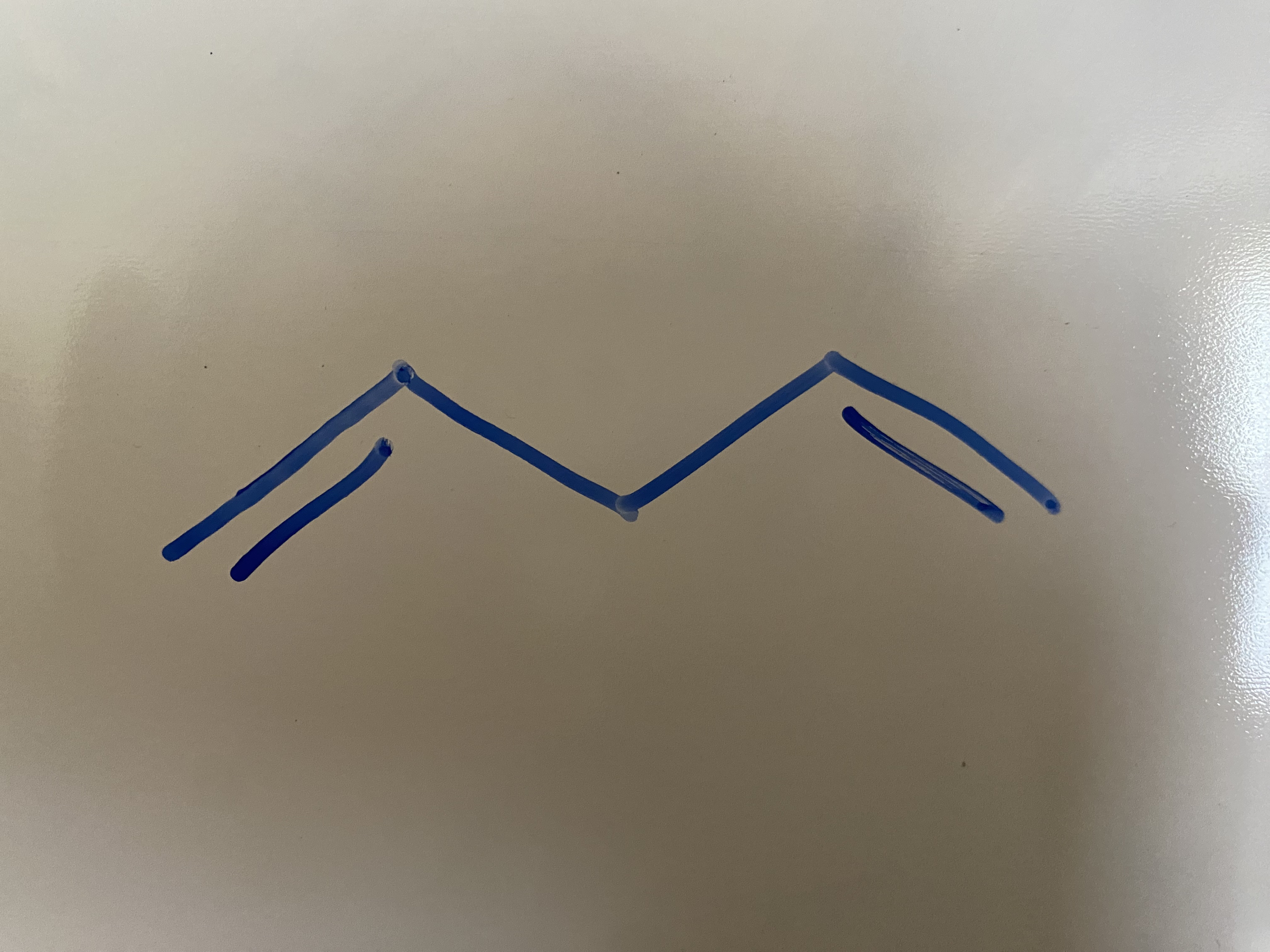
-253 kJ/mol

-225 kJ/mol