Organic Chemistry Reaction and Carbocation Flashcards
1/54
Earn XP
Description and Tags
These flashcards cover key concepts related to organic chemistry reactions, carbocation stability, and rearrangements, including definitions, mechanisms, and reagents.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
55 Terms
Alkane radical halogenation
Free radical substitution where an alkane C-H is replaced by C-X; involves Cl2 or Br2 and heat.
Allylic bromination
Radical substitution at the allylic position of an alkene using NBS, resulting in allylic bromide.
Radical HBr Addition
Anti Markovnikov addition of HBr to an alkene via radical mechanism, resulting in an alkyl bromide.
SN1 reaction
Two-step substitution mechanism via a carbocation; favored by tertiary substrates and weak nucleophiles.
SN2 reaction
One-step backside substitution mechanism; favored by methyl to primary substrates and strong nucleophiles.
E1 reaction
Two-step elimination mechanism via a carbocation; similar substrate preference to SN1.
E2 reaction
One-step, base-promoted elimination mechanism; requires strong bases and stereochemical anti periplanar arrangement.
Hydrohalogenation of alkene
Electrophilic addition of HX across an alkene via carbocation; follows Markovnikov's rule.
Acid catalyzed hydration
Addition of water across a double bond using acid; follows Markovnikov's rule with possible carbocation rearrangement.
Oxymercuration demercuration
Markovnikov hydration of an alkene with no rearrangement, involving Hg(OAc)2 and NaBH4.
Hydroboration Oxidation
Anti Markovnikov hydration of alkene resulting in an alcohol; involves BH3 and H2O2.
Halogenation of alkene
Anti addition of two halogens to a double bond, resulting in vicinal dihalides.
Halohydrin formation from alkene
Formation of a halogen and alcohol from an alkene, typically with anti addition.
Catalytic hydrogenation of alkene
Reduction of a double bond to an alkane via metal-catalyzed addition of H2.
Epoxidation of alkene
Formation of an epoxide from an alkene using a peroxyacid.
Anti dihydroxylation via epoxide
Formation of trans 1,2 diol from an alkene using an epoxide intermediate.
Syn dihydroxylation of alkene
Direct syn addition of two OH groups across a double bond to form 1,2 diol.
Ozonolysis of alkyne
Oxidative cleavage of an alkyne to form carbonyl compounds, aldehydes, or ketones.
Dehydrohalogenation to form alkyne
Formation of an alkyne from vicinal or geminal dihalide via two E2 eliminations.
Terminal alkyne alkylation
Formation of a new carbon-carbon bond using a terminal alkyne and primary alkyl halide.
Full hydrogenation of alkyne
Reduction of an alkyne to an alkane through the addition of H2.
Lindlar reduction of alkyne
Partial hydrogenation of an alkyne to form a cis alkene.
Dissolving metal reduction of alkyne
Partial reduction of an alkyne to yield a trans alkene.
HX addition to alkyne
Electrophilic addition of hydrogen halides to an alkyne, following Markovnikov's rule.
Carbocation stability order
Stability of carbocations ranks as tertiary > secondary > primary > methyl.
Resonance stabilized carbocations
Carbocations that are stabilized by the delocalization of charge through resonance.
Very unstable carbocations
Carbocations that rarely form due to their high energy; includes vinylic and aryl carbocations.
Reactions that can rearrange
Mechanisms with carbocation intermediates where rearrangement is possible, e.g., SN1 and E1.
Reactions that do not rearrange
Mechanisms avoiding carbocations, such as SN2 and E2, where rearrangement is not expected.
Hydride shift
Carbocation rearrangement involving the movement of a hydride to stabilize the carbocation.
Alkyl (methyl) shift
Carbocation rearrangement involving the migration of an alkyl group to stabilize the positive charge.
Ring expansion via carbocation
Rearrangement that changes ring size to reduce strain and stabilize the carbocation.
Rearrangement checklist
A method to determine if a carbocation rearrangement is likely based on mechanism and structure.
Ozonolysis of alkene (reductive workup)
Cleavage of an alkene using O3 followed by a reductive workup (e.g., DMS or Zn/H2O) to yield aldehydes and ketones.
Ozonolysis of alkene (oxidative workup)
Cleavage of an alkene using O3 followed by an oxidative workup (e.g., H2O_2) to yield carboxylic acids from aldehyde precursors and ketones.
Ozonolysis of alkyne
Cleavage of an alkyne using O_3 followed by an oxidative workup to form carboxylic acids (from terminal and internal alkynes) or diketones if specifically designed.
Products of reductive ozonolysis (alkene, R_2C=)
A disubstituted carbon of an alkene double bond (R2C=) yields a ketone (R2C=O) after reductive ozonolysis (O3, DMS or Zn/H2O).
Products of reductive ozonolysis (alkene, RHC=)
A monosubstituted carbon of an alkene double bond (RHC=) yields an aldehyde (RHC=O) after reductive ozonolysis (O3, DMS or Zn/H2O).
Products of reductive ozonolysis (alkene, H_2C=)
An unsubstituted carbon of an alkene double bond (H2C=) yields formaldehyde (H2C=O) after reductive ozonolysis (O3, DMS or Zn/H2O).
Products of oxidative ozonolysis (alkene, R_2C=)
A disubstituted carbon of an alkene double bond (R2C=) yields a ketone (R2C=O) after oxidative ozonolysis (O3, H2O_2).
Products of oxidative ozonolysis (alkene, RHC=)
A monosubstituted carbon of an alkene double bond (RHC=) yields a carboxylic acid (RCOOH) after oxidative ozonolysis (O3, H2O_2).
Products of oxidative ozonolysis (alkene, H_2C=)
An unsubstituted carbon of an alkene double bond (H2C=) yields carbon dioxide (CO2) after oxidative ozonolysis (O3, H2O_2).
Products of ozonolysis of internal alkynes
An internal alkyne (RC \equiv CR') is cleaved by ozonolysis (O3, H2O) to form two carboxylic acids (RCOOH and R'COOH).
Products of ozonolysis of terminal alkynes
Carboxylic Acid and Carbon Dioxide
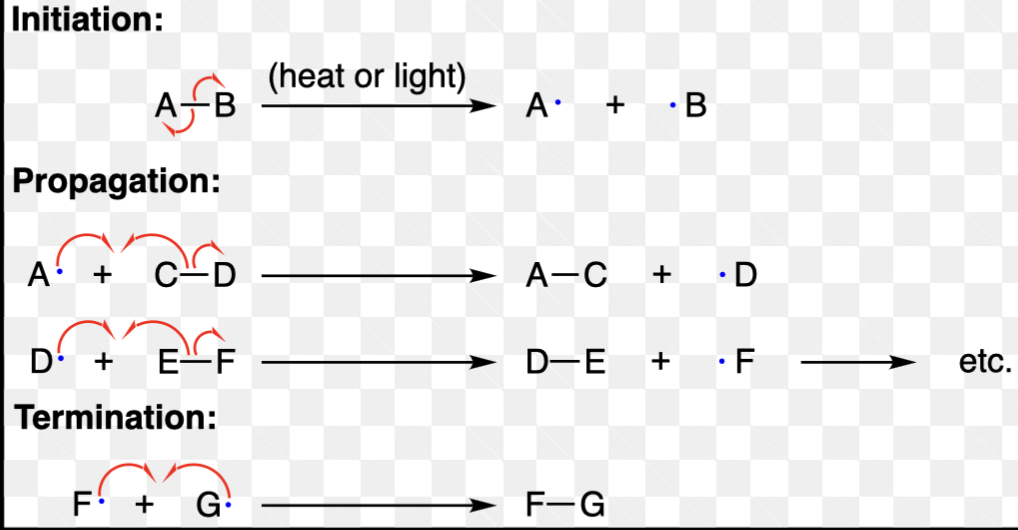
Radical Reaction Steps
Radical Reaction Steps
Radical Initiation
The first step in a radical reaction where a stable molecule (often an initiator) breaks apart to form free radicals, which can then propagate the reaction.
Radical Propagation
The series of steps in a radical reaction where the free radicals react with stable molecules to form new radicals, continuing the chain reaction.
Radical Termination
The final step in a radical reaction where two free radicals combine to form a stable product, effectively stopping the reaction.
Radical Initiation
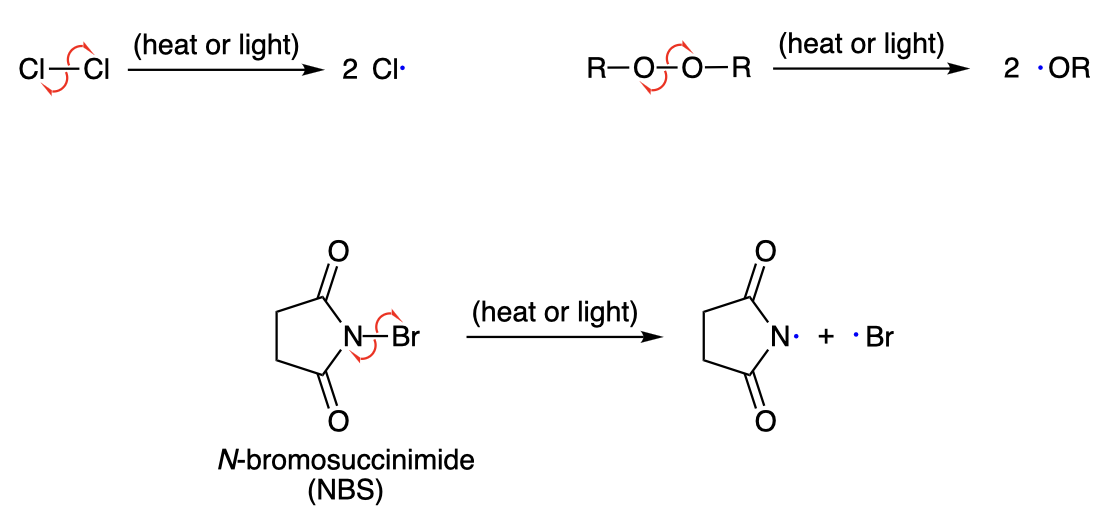
Radical Propagation
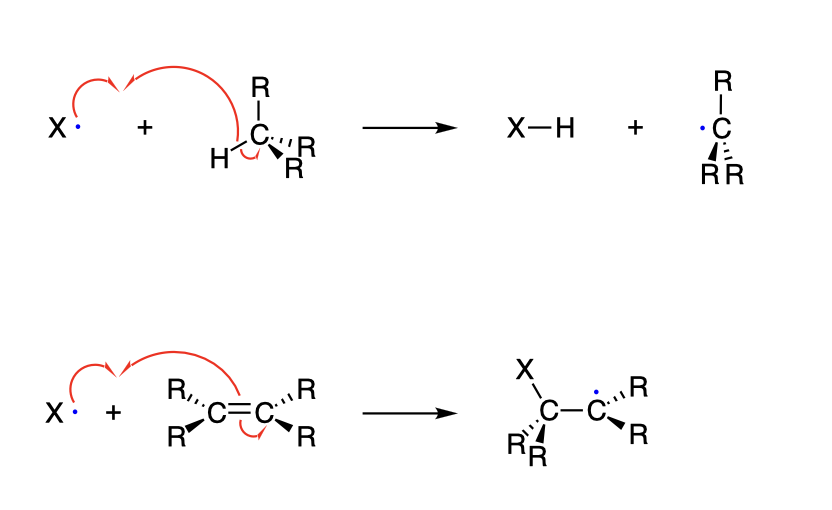
Radical Termination
Difference between E1 and E2 reactions
E1 is a two-step elimination via a carbocation intermediate (similar to SN1, favored by tertiary substrates), while E2 is a one-step, concerted elimination requiring a strong base and an anti periplanar arrangement of the reacting groups.
Difference between E2 and SN2 reactions
E2 is a one-step elimination reaction favored by strong bases, resulting in an alkene, requiring anti periplanar geometry. SN2 is a one-step substitution reaction favored by strong nucleophiles, leading to inversion of configuration at a stereocenter, preferred for methyl to primary substrates.
Difference between SN1 and E1 reactions
Both SN1 (substitution) and E1 (elimination) are two-step reactions that proceed via a carbocation intermediate and are favored by tertiary substrates. SN1 replaces a leaving group with a nucleophile, while E1 removes elements to form a new pi bond.