AP Bio: Unit 1 - The Chemistry of Life
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
What are the major chemical elements that make up life? (Topic 1)
Carbon
hydrogen
oxygen
phosphorus
sulfur
What are isotopes and how are they used in biology? (Topic 1)
Isotopes - atoms of the same element with different amounts of neutrons
Can be used to track compounds, determine ages of atoms, create images of biological processes
Why and how is carbon used in organic molecules? (Topic 1)
Can bond to up to four other molecules
Allows for the creation of unique/complex molecules
Used in organic molecules as the backbone
What types of organic molecules contain nitrogen? (Topic 1)
Amino acids
proteins
nucleic acids
ATP
What types of organic molecules contain phosphorus? (Topic 1)
nucleic acids (mainly)
lipids
How does carbon, nitrogen and phosphorus cycle between living and non-living reservoirs and what factors affect these cycles? (Topic 1)
Carbon cycle processes
photosynthesis
cellular respiration
decomposition → carbon released into atmosphere or soil
carbon reserves (ocean, atmosphere, fossel fuels)
Nitrogen cycle processes
nitrogen fixation - converting atmospheric nitrogen into biologically available forms (ammonia, NH3) by ntrogen-fixing bacteria (rhizobium) or lightning
nitrogen-fixing bacteria in soil forming symbiotic relationships with plants
nitrification - process where ammonia (NH3) is oxidized into nitrite (NO2-) and then nitrate (NO3-) by nitrifying bacteria for plant uptake
denitrification - reducing nitrate back into atmospheriic nitrogen by denitrifying bacteria
Phosphorus cycle processes
weathering - physical/chemical breakdown of rocks and minerals → phosphorus released as PO4- into soil and water
erosion - weathered materials transported by wind, water, ice to other places
when plants and microorganisms absorb PO4- from soil or water and incorporate them into biomass
sedimentation - phosphorus-containing particles settle at the bottom of aquatic systems to form sedimentary layers
Factors affecting these cycles
natural forces
human-driven forces
What functional group determines an amino acid? (Topic 2)
R group
What are common chemical function groups that are part of biological molecules? (Topic 2)
Hydroxl (-OH)
amino (-NH2)
carbonyl (C=O)
phosphate (-PO4)
sulfhydrl (-SH)
methyl (CH3)
How do the subcomponents of biological molecules and their sequence determine the properties of that molecule? (Topic 2)
The subcomponents and their sequence determine the structure and thus function of biological molecules
Monomers of macromolecules
Proteins - amino acids (bond together with peptide bonds)
Nucleic acids - nucleotides
Carbohydrates - monosaccharides
Lipids - fatty acids
Saturated - solid at room temp. due to no double bonds
unsaturated - liquid at room temp. due to double bonds
What is a polymer? (Topic 2)
Large molecules made up of repeatiing small units called monomers that are linked through chemical bonds
What are hydrolysis and condensation reactions? (Topic 2)
Hydrolysis - when a hydrogen atom is added into a large molecule causing it to break apart into smaller molecules
Condensation - when a hydrogen atom is taken away in order to bond smaller molecules together to create a larger molecule (used to create macromolecules from monomers)
What is the chemical make-up of carbohydrates, and why do different carbohydrates like starch and cellulose have unique structures and functions? (Topic 2)
Carbohydrates are made up of carbon, hydrogen, and oxygen in a 1:2:1 ratio (think glucose
The differences in linkage lends to differences in structure and function
Starch stores glucose in plants
Cellolose provides structure and support in plants (cannot be digested by humans)
What is the general structure of lipids? (Topic 2)
Lipids are made up of a glycerol backbone, 2 fatty acid tails (hydrophobic), and a phosphate group (hydrophilic) (used to create phospholipid bilayer/cell membrane)
How does the different parts of a phospholipid’s structure interact with other molecules to produce its function properties? (Topic 2)
Phosphate head interacts with/is attracted to water (hydrophilic)
Fatty acid tails repel water (hydrophobic)
Creates bilayer
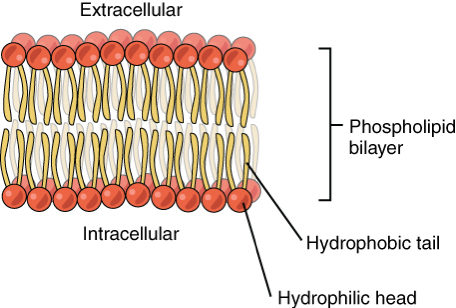
What is hydrophobicity and polarity? (Topic 2)
Hydrophobicity - aversion to water
Polarity - measure of how electrically charged a molecule is
Small, nonpolar molecules can easily pass through phospholipid bilayer through osmosis
What does it mean that an amino acid chain has directionality? (Topic 2)
It has two ends that are chemically distinct from one another
A free amino group (amino terminus/N-terminus)
A free carboxyl group (carboxyl terminus/C-terminus)
What are the four levels of protein structure? (Topic 2)
Primary - the sequence of amino acids in a polypeptide chain (straight line, no folding
Secondary - Folded structure that form within a polypeptide due to interactions between atoms of the backbone
Does not involve R group atoms
Most common - a helix and b pleated sheet held in shape by hydrogen bond
Tertiary - overal three-dimensional structure of a polypeptide
Due to interactions between r groups of the amino acids like hydrogen bonding, ionic bonding, non-covalent bonds in general
Disulfide bonds = strongeset bonds that contribute to tertiary structure (covalent linkages between sulfur-containing side chains)
Quaternary structure - When proteins are made up of multiple polypeptide chains (subunits), and these chains come together
Basically consists of more than one amino acid chain
What makes up a nucleotide? (Topic 2)
A phosphate group
A sugar molecule (pentose)
A nitrogenous base
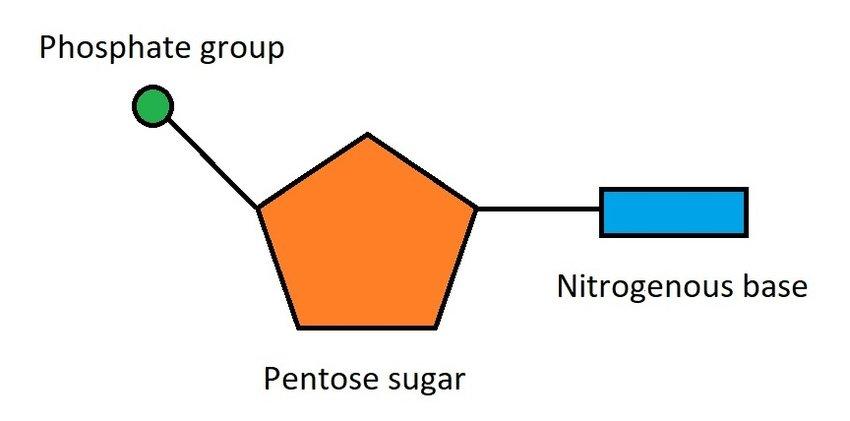
How do nucleotides link together to form nucleic acids? (Topic 2)
Through covalent bonds between the phosphate group of one nucleotide and the sugar of another
What determines directionality of a nucleic acid chain? (Topic 2)
The two ends of a polynucleotide chain are different from each other
5’ end (the beginning) is where the 5’ phosphate group of the first nucleotide sticks other
3’ end (the end) is where he 3’ hydroxyl of the last nucleotide is exposed
DNA sequences run from 5’ to 3’
New nucleotides are added to a strand of DNA/RNA
strand grows from 3’ end allowing the 5’ phosphate of incoming nucleotides to attach
What chemical interactions occur to create a DNA double-helix? (Topic 2)
Hydrogen bonds between complementary nitrogenous base pairs and covalent bonds within each strand
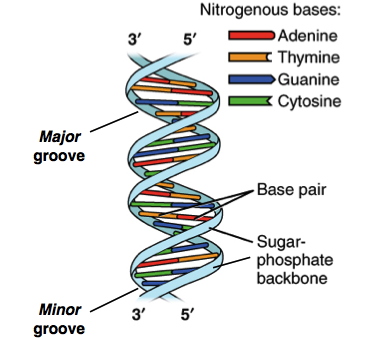
What are the structural similarities and differences between DNA and RNA? (Topic 2)
Similarities
Both composed of nucleotides
Both share adenine, guanine, and cytosine
Both are composed of long chains of nucleotide monomers linked together
A pairs with T/U and G pairs with C in both
Differences
The sugar in DNA is deoxyribose
The sugar in RNA is ribose
DNA has thymine
RNA has uracil
DNA is typically double stranded
RNA is typically single stranded
DNA is more stable
RNA is less stable and more susceptible to degradation
Why is water a polar molecule, and what are hydrogen bonds? (Topic 3)
It is polar because of the uneven distrbution of elections within the molecule which creates a partial positive charge on the hydrogen atoms and a partial negativec charge on the oxygen atoms
Hydrogen bonds are attraction between a hydrogen atom and an electronegative atom (oxygen, nitrogen, or fluoriine) in another or within the same molecule
What are the properties of water? (Topic 3)
Universal solvent
High specific heat
Hight surface tension
less dense in solid form than in liquid form due to expansion
Polarity gives way to cohesive and adhesive properties
What is osmosis and water potential? (Topic 3)
Osmosis - the flow of a solvent from high solute concentration to low solute concentration through a semipermeable membrane
Water potential - the likelihood of water flowing from one place to another
What is transpiration, and what are the environmental factors that influence it? (Topic 3)
Transpiration - evaporation of water from plants through stomata
Temperature can increase rate
Humidity can lower rate
Air movement can increase rate
Light intensity can increase rate