POLYMERS AND CRYSTALLINITY - BME 296 EXAM 1
1/64
Earn XP
Description and Tags
lectures 5-7
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
65 Terms
polymer
a chain of monomers
n signifies the number of repeating units
number of repeating units in a polymer is called degree of polymerization
polymer size is the molecular weight of the chain
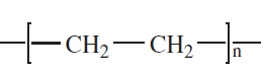
molecular weights of polymers - example
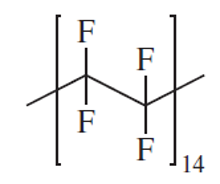
Determine molecular weight of poly tetrafluoroethylene(Teflon) (C2F4)n
2 carbon atoms and 4 fluorine atoms per repeat unit
First determine atomic mass of individual components (will be provided)
Atomic mass of carbon = 12 g/mole
Atomic mass of fluorine = 19 g/mole
2(12 g/mole) + 4(19 g/mole) = 100 g/mole/repeat unit
Since there are 14 repeat units in this polymer chain, the molecular weight of the polymer equals 14 times the molecular weight of the repeat unit, plus 2 hydrogen atoms, one to terminate each end of the polymer chain:
100 g/mole/repeat unit 14 repeat units + 2 hydrogen atoms (1 g/mole hydrogen) = 1,402 g/mol
molecular weights of polymers
During synthesis, we can get polymers that have a distribution of molecular weights in various individual components
It is important to take these individual components into account.
Number-average molecular weight (Mn) and weight-average molecular weight (Mw)
difference between Mn and Mw
number average molecular weight refers to the mole fraction of molecules in a polymer sample whereas the weight average molecular weight is the weight fraction of molecules in a polymer sample {moles versus mass}
how to find number-average molecular weight Mn
The number-average molecular weight is found by dividing the chains into a series of size ranges and computing the fraction of chains with that size. This can be written
Ni is the number of chains with molecular weight Mi
Mi represents an average molecular weight for the chosen molecular weight range
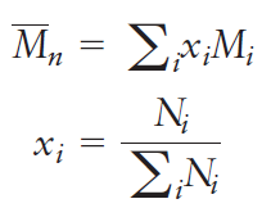
how to find weight-average molecular weight Mw
The weight-average molecular weight is calculated using the weight fraction of the chains within the selected size ranges
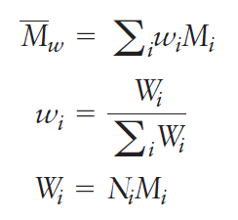
polydispersity index (PI)
the ratio of the two molecular weights
smallest possible value is 1
it is a measure of the heterogeneity of a sample based on size
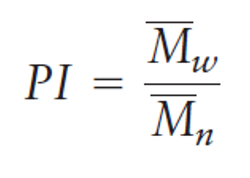
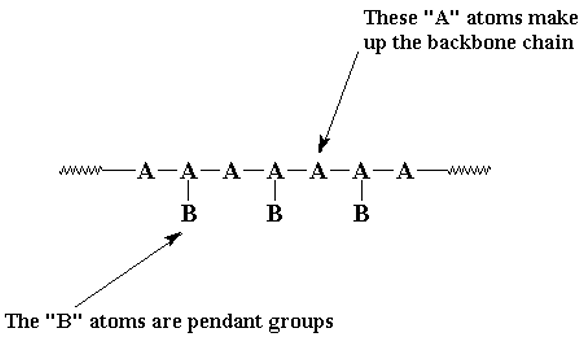
arrangement of molecules in polymers
linear structure made up of A atoms - backbone
some atoms in chain may have atoms attached to them called pendant groups
Simplest polymer examples are hydrocarbons (contain hydrogen and carbon)
set of polymers are vinyl polymers (Polymers made of small molecules containing carbon-carbon double bonds)
Backbone of polymer usually repeats itself
carbon atoms in polymers
higher number of carbon atoms - better mechanical properties
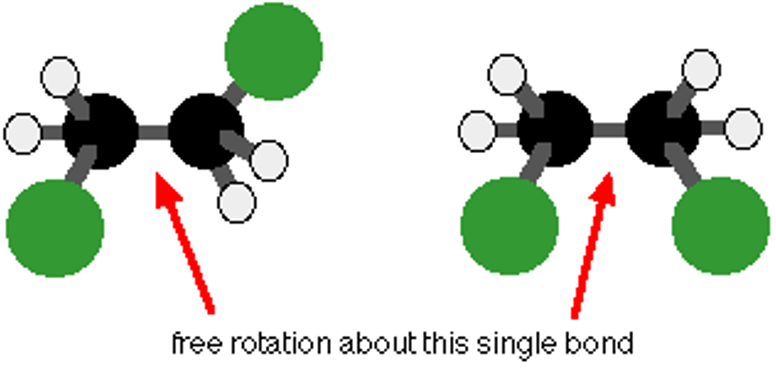
rotation of bonds
carbon-carbon single bonds can rotate but covalent bonds cannot
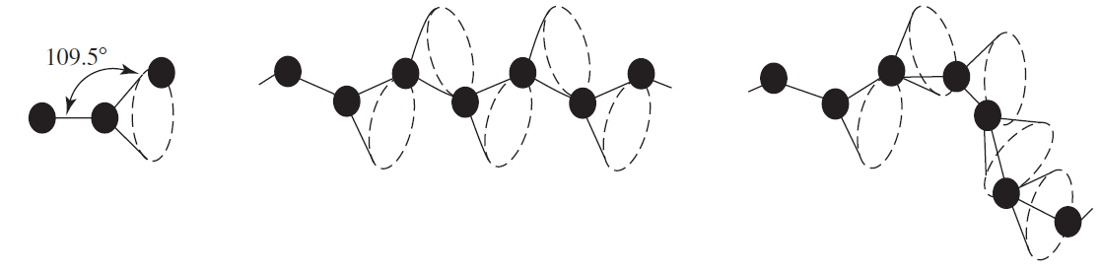
configuration of monomers
polymers can fold back on themselves, containing large bends and kinks playing a large role in mechanical properties
polymer structure
Linear end-to-end
Branched - may have chains that branch off from main chain
Cross linking - increase molecular weight of the polymer as they are bonded together increasing viscosity
thermo and thermoset polymers
linear and branched thermo - heat changes shape and can be molded
cross linked thermoset - once set cannot be molded again
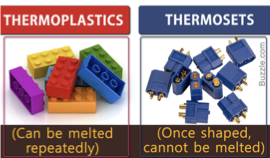
how to differentiate branched and cross-linked?
Depends on extent to which the side-chains on the backbone link adjacent chains. - - -
Best way to test? Dissolve in solvent
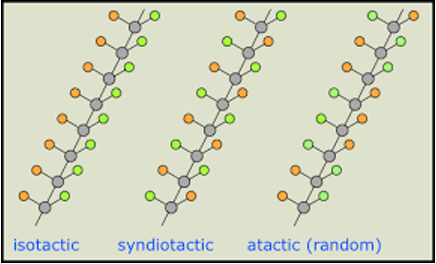
configuration of polymers - tacticity
can be isotactic, syndiotactic, and atactic
“R” represents a generic side group or atom
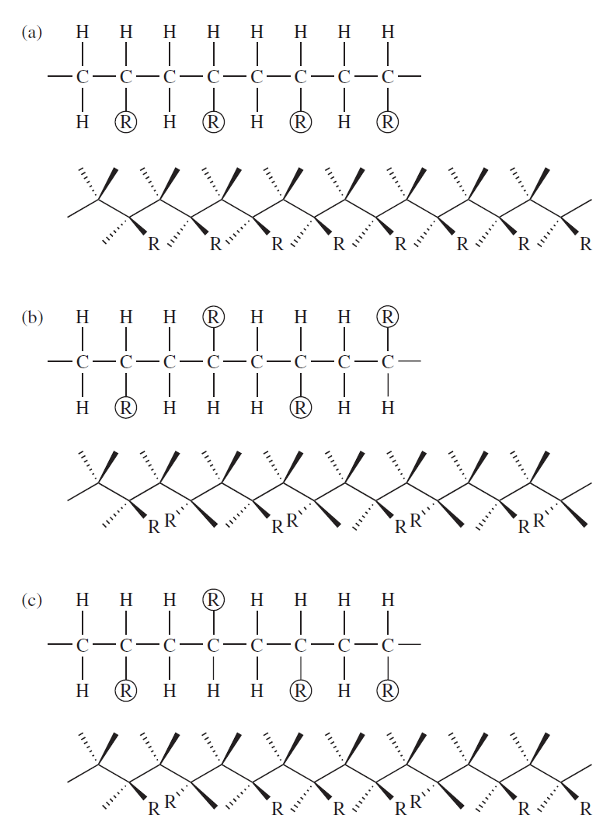
isotactic configuration
R groups are arranged on the same side of the chain
syndiotactic configuration
R groups alternate positions on either side of the chain
atactic configuration
R groups are situated randomly
crystallinity in polymers
the amount of long range “order” in a polymer determines its crystallinity
more crystalline, more aligned chain
increased crystalline, more strength
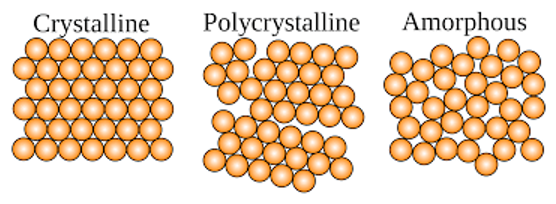
degree of crystallinity affects…
mechanical properties
crystallinity in polymers - branching and bulky side groups
branched polymers are usually less crystalline than linear ones
bulky side groups along the polymer chain as well as high degrees of polymer branching impede the ability of the chains to reside close enough to one another to form crystal structures
with increasing size of the side groups, it becomes progressively more difficult for the polymer to fold and align itself along the crystal growth direction
bulky group: a group that contains more than 2 atoms
benzene is pretty common side group
tacticity plays a role in crystallinity
Greater the order in a polymer, the greater the likelihood for it to undergo crystallization
molecules prefer an ordered arrangement with maximum packing density to maximize the number of secondary bonds
Isostatic is more crystalline than syndiotactic, which is more crystalline than atactic.
atactic polymers are usually not crystallizable and are mostly amorphous.
what determines crystallinity?
isotactic > syndiotactic > atactic
linear > branched
small pendant groups > large pendant groups
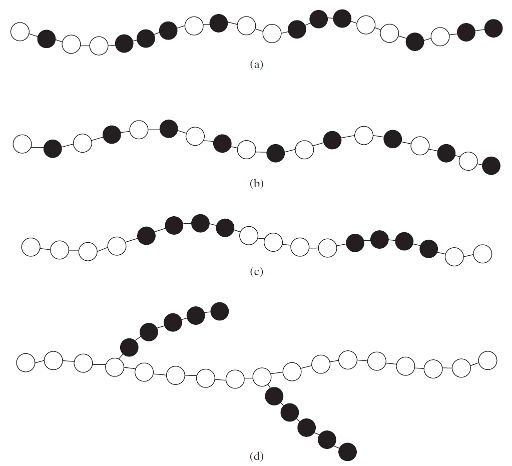
copolymers
Random copolymer - the two monomer units are distributed along the chain with no pattern.
Alternating copolymer - monomer types alternate.
Block copolymer - each type of repeat unit is clustered in regions (blocks) along the chain.
Graft copolymer - homopolymer chains are attached to a main homopolymer chain containing a different repeat unit.
mechanism behind synthesis of polymers
addition, condensation, and genetic engineering
addition polymerization
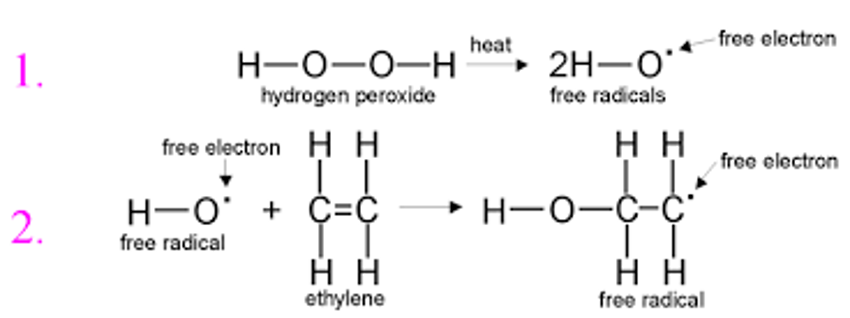
Initiation: Requires activation of a monomer through reaction either with a radical species for free radical polymerization or an anionic or cationic species for anionic and cationic polymerization
Example: Can be initiated by thermal decomposition of a peroxide to produce free radicals
The free radical breaks the C=C bond by joining one side of the monomer allowing other side of monomer free to react with another molecule
Propagation: Monomers continue to successively join the polymer chain and increase its molecular weight
Free radical keeps adding to chain end
termination
In the case of free radical polymerization, termination occurs through destruction of the active site by the reaction of free radicals.
Adding a thiol to stop reaction
Another method involves the reaction of the activated end on two propagating chains to form a longer chain, thus ending the growth of both chains
condensation polymerization
Usually involves more than one monomer species, and no radical initiator is required.
The polymerization occurs through elimination of one molecule (usually water, another example is methanol).
polymer production via genetic engineering
Provides more control over the architecture and weight distributions
Cannot create synthetic polymers, only natural ones (correction, next slide)
This method involves the expression within a host organism (usually bacteria) of a genetic vector that encodes the protein polymer of interest.
This can be achieved by one of two general methods.
First, DNA encoding the protein polymer can be isolated from an organism that naturally produces it, and the DNA coding units can be introduced into the DNA of the host bacteria for expression and polymer production.
Alternatively, the DNA encoding the protein polymer can be chemically synthesized and subsequently introduced into the host organism.
The second method is more widely used and allows for a high level of control over the sequence of the protein polymer
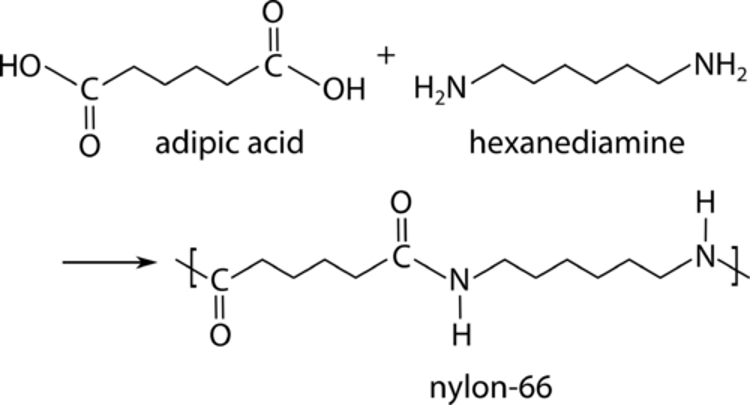
bulk polymerization
Simplest technique
only monomer and a monomer-soluble initiator are present in the reaction.
Add heat, agitation if needed
Polymer is precipitated out
Advantage: High purity, no need to filter anything. Can directly be made in a mold.
Disadvantage: These reactions are exothermic. Especially when large quantities are required.
Example: PMMA or bone cement
solution polymerization
Conducting the polymerization reaction in water or an appropriate organic solvent with high thermal conductivity
Important that the monomer and initiator be soluble in the chosen solvent and that the solvent can be easily recovered following the reaction.
May add catalyst if needed
Advantage: Heat conducted.
Disadvantage: it has a small polymer yield per reaction volume and requires an additional step to remove the solvent, if needed
Same as bulk polymerization except final polymer will be in solution.
E.g. Paint in toluene
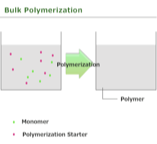
suspension polymerization
Monomer and initiators that are not soluble in water are added under stirring to a reactor full of water (mechanical agitation).
The insolubility of the monomer coupled with the mechanical agitation leads to the formation of monomer droplets containing initiator (which is soluble in monomer).
After the reaction, the resulting polymer beads can be recovered through filtration and washed.
Essentially bulk polymerization is carried out in suspended droplets
Monomer and product must be insoluble in water
Agitation causes increase in viscosity of bead and that increases reaction rate.
Size of droplets depends on ratio of water/monomer, mode and speed of agitation
Usually get spherical beads or pearls
Ex: Polystyrene beads in Styrofoam made this way.
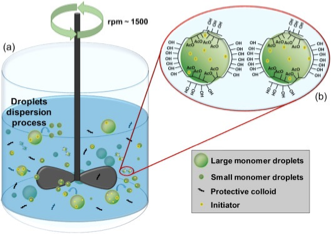
emulsion polymerization
Starts with an emulsion of water, monomer and surfactant
You get small particles of polymers like nanoparticles
Most common type is oil-in-water emulsion in which droplets of monomer (the oil) are emulsified (using surfactant) in water
Involves the addition of a hydrophobic monomer, a slightly soluble or insoluble in water initiator and a surfactant (emulsifier) to a reactor containing water.
Emulsion polymerization results in the formation of polymer beads or rods, depending upon the reaction conditions and the surfactant used.
Used when you have a hydrophobic polymer
difference between suspension and emulsion
Suspension polymerization is a mechanical process
Emulsion polymerization is a chemical process which requires a surfactant to make the monomer "emulsify."
mechanism of polymer formation - process of polymer formation
Bulk and solution need soluble in solvent
Suspension and emulsion non-soluble in solvent
carbon-based materials
Considered ceramic- graphite. It does not posses a standard unit cell, still its crystalline.
The structure consists of planes of hexagonally arranged carbon atoms.
Within the planes, each carbon atom is bonded covalently to three neighbors, while the fourth valence electron participates in van der Waals interactions with the plane above it.
An important property of graphite is that it can absorb gases. This is used to prepare pyrolytic carbon
Pyrolytic carbon is where carbon in the gaseous state is deposited onto another material
Pyrolytic carbon has been used extensively in cardiovascular applications due to its properties
Biocompatible, i.e. does not elicit any adverse reactions when implanted into human bodies
Thromboresistant i.e. resists blood clotting
Good durability
Good wear resistance
Good strength
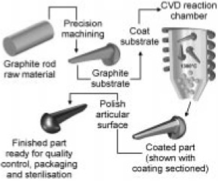
carbon based materials failed due to..
fatigue and fracture (think heart valve fracture from review)
material characterization
Characterization of properties relating to the chemical composition of a biomaterial can be accomplished through two major techniques, spectroscopy and chromatography.
In spectroscopy, one measures how compounds absorb different types of energy.
The absorbed energy results in excitation of the sample by creating changes in electron or mechanical motion within the molecule.
Chromatography uses various means to physically separate molecules on the basis of chemical characteristics such a charge or molecular weight.
x-ray diffraction (XRD)
X-ray diffraction is commonly used to determine structures of crystals, including calculation of Miller indices and unit cell size
Because the wavelength of X-rays (0.5–50Å) is similar to the distance between atoms in a solid, they are ideal for exploring atomic arrangement in crystal structures.
principles:
X-rays are produced by heating a filament in a cathode ray tube. This produces electrons that are accelerated towards a target
The electrons have high energy and displace inner shell electrons of target material producing characteristic X-ray spectra.
Waves in alignment constructive interference and signal is amplified
Waves not in alignment destructive interference and signal is lost
When X ray hits electrons, energy is increased but not enough to displace electrons
New X ray emitted with same wavelength from electron (Elastic scattering)
bragg’s law
what information can we get?
Identify crystalline phases and orientation
Determine structural properties:
Lattice parameters
Strain
Grain size
Determine atomic arrangement
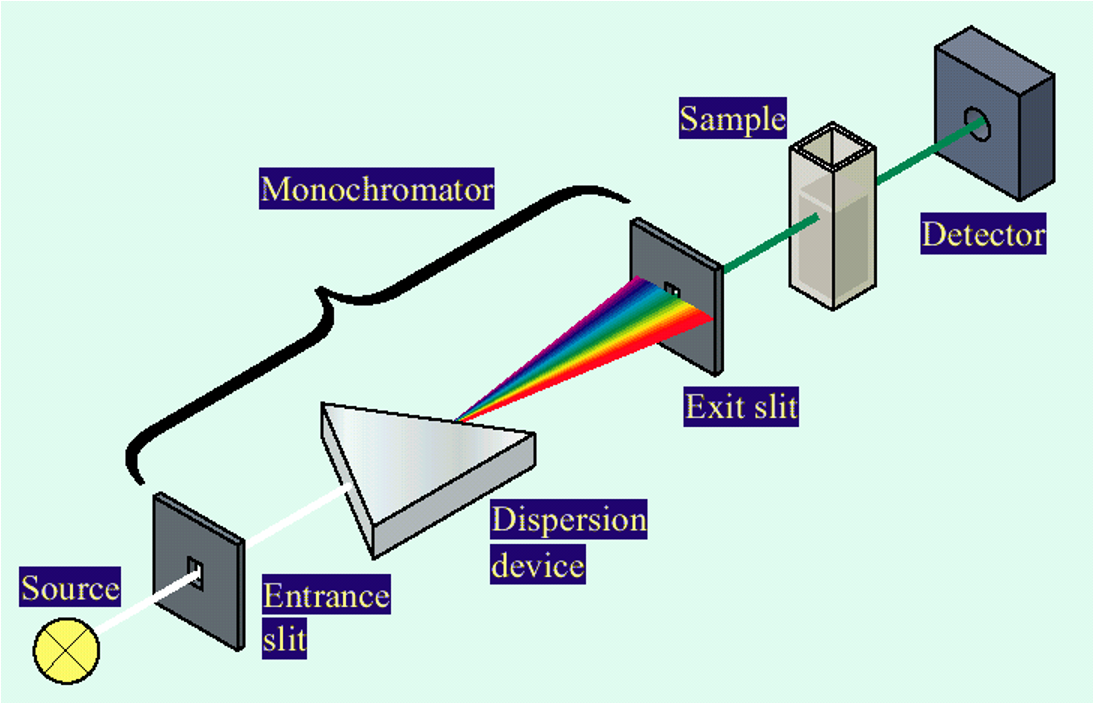
ultraviolet and visible light spectroscopy (UV-Vis)
quantitative technique used to measure how much a chemical substance absorbs light.
Measure the intensity of light that passes through a sample with respect to the intensity of light through a reference sample or blank.
Lambert Beer law
states that there is a linear relationship between the concentration and the absorbance of the solution, which enables the concentration of a solution to be calculated by measuring its absorbance.
higher concentration - more light absorbed
The absorption of UV-VIS radiation [wavelength approximately 185–1100 nm] by a molecule (M) may promote one or more of its valence electrons to a higher energy state, resulting in molecular excitation to a new state (M*)
M + hv = M*
Where v is the frequency of radiation and h is the plank’s constant (6.6X10^-34J-s)
After excitation, the molecule returns to its ground state through the conversion of the excitation energy to another form, such as thermal energy (heat).
This process is known as relaxation
M* = M + heat
Calculate light absorbed. You know how much was sent in, detect how much was transmitted.
Difference is light absorbed.
what information can we get?
Produce spectra to help identify samples or chemical groups within samples.
UV-VIS is often used to quantify the amount of a certain compound in a mixture or solution
Concentrations of both inorganic and organic substances can be quantified using this method, but it is more commonly used in the biomaterials field for organic materials
![<ul><li><p><span>quantitative technique used to <strong>measure how much a chemical substance absorbs light</strong>.</span></p></li><li><p><span>Measure the intensity of light that passes through a sample with respect to the intensity of light through a reference sample or blank.</span></p></li><li><p><span>Lambert Beer law</span></p><ul><li><p style="text-align: left"><span>states that <strong>there is a linear relationship between the concentration and the absorbance of the solution</strong>, which enables the concentration of a solution to be calculated by measuring its absorbance.</span></p></li><li><p>higher concentration - more light absorbed</p></li></ul></li><li><p><span>The absorption of UV-VIS radiation [wavelength approximately 185–1100 nm] by a molecule (<em>M</em>) may promote one or more of its valence electrons to a higher energy state, resulting in molecular excitation to a new state (M*)</span></p><ul><li><p>M + hv = M*</p></li><li><p><span>Where v is the frequency of radiation and h is the plank’s constant (6.6X10^-34J-s)</span></p></li></ul></li><li><p><span>After excitation, the molecule returns to its ground state through the conversion of the excitation energy to another form, such as thermal energy (heat).</span></p></li><li><p><span>This process is known as <strong>relaxation</strong></span></p><ul><li><p>M* = M + heat</p></li></ul></li><li><p><span>Calculate light absorbed. You know how much was sent in, detect how much was transmitted.</span></p></li><li><p><span>Difference is light absorbed.</span></p></li></ul><p>what information can we get?</p><ul><li><p><span>Produce spectra to help identify samples or chemical groups within samples.</span></p></li><li><p><span>UV-VIS is often used to quantify the amount of a certain compound in a mixture or solution</span></p></li><li><p><span>Concentrations of both inorganic and organic substances can be quantified using this method, but it is more commonly used in the biomaterials field for organic materials</span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/16a41853-1179-403d-8a40-4999b9b83046.png)
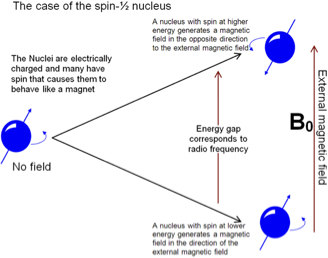
nuclear magnetic resonance spectroscopy (NMR)
utilizes radiation in the radio-frequency region (0.5–75 m) to excite molecules.
induces changes in the nucleus, rather than the electrons, of an atom- using spin of nucleus
The presence of a strong magnetic field is needed to observe the nuclear transitions
If molecules are placed in a strong magnetic field, the nuclei of some atoms will begin to behave like small magnets.
When radio frequency waves are applied to the sample, the nuclei will begin to resonate at their own specific frequencies.
Odd number of protons means a nucleus will have spin, even no-spin
The resonant frequencies of the nuclei are then measured
The height of each peak represents the number of nuclei that resonates at each specific frequency. This is known as the intensity of signal. The more resonating nuclei, the higher the intensity.
The value of each frequency, or tone, gives information about the surroundings of the atom such as its neighboring atoms and their relative positions.
Nuclei that have odd number of protons and/or neutrons have spin
what information can we get?
NMR spectra are generally plotted as peak intensity (y-axis) versus chemical shift in parts per million (x-axis, ppm).
Samples can be examined for the presence of certain types of bonds using characteristic chemical shifts for different groups
Also, the relative amounts of atoms in various chemical states can be easily obtained.
mass spectrometry
Determines the atomic or molecular masses of various species in a material (Mass/charge ratio)
The sample is first ionized though bombardment with high-energy particles (usually electrons).
The resulting charged species are forced through a magnetic field, which interacts with them to deflect them from a linear path.
Because lighter species are deflected more than heavier ones, the particles can be separated based on mass
Can distinguish between isotypes of same element
Isotopes are atoms that have the same atomic number but different mass numbers due to a change in the number of neutrons
what information can we get?
Mass spectrometry can provide qualitative and quantitative assessment of both inorganic and organic molecules,
for biomaterials it is primarily used for analysis of natural and synthetic polymers
Isomers structural (look at fragments), compare against what we already have
crystallinity and polymeric biomaterials
Depends on chemical structure of mer and polymer
The following factors can influence percent crystallinity:
mer side groups (bulky side groups reduces crystallinity)
chain branching (more branching reduced crystallinity)
Tacticity (isotactic highest crystallinity)
regularity of mer placement in copolymers (more regular better crystallinity)
Molecular weight (generally increased molecular weight reduces crystallinity)
molecular weight, mechanical properties and crystallinity
Higher molecular weight reduces crystallinity in a polymer because as the chains become longer, they become more entangled with each other, limiting their mobility and making it harder for them to align and pack together in the ordered arrangement required for crystal formation; essentially, the increased entanglement restricts the chains' ability to move into a crystalline structure
crystallinity and how it changes properties
changes mechanical and physical properties
changes interactions with cells
temperature and its effects on polymers
Increase in heat causes molecules to have more kinetic energy and move; opposite true when temp decreased
Polymers can change mechanical properties based on temperature.
Therefore, this temperature-property relationship is a good indicator to determine whether polymer can be used as a hard or soft tissue device
thermal transition
Molecules transition from one phase to another when thermal energy is added called thermal phase transition or thermal transition
thermal transition in biomaterials
Thermal transition points are based on how materials deform
Important to investigate as biomaterials at temperatures they will be used:
Examples of high temperatures: Nano particles in cancer cells to be used with imaging/therapy
Examples of low temp: External stitches in cold weather
Example of when we need biomaterial to change property in different environments: To increase ease of use, some collagen scaffolds are liquid when outside the body so that it can be easily injected. It becomes solid once inside body
thermal transitions: melting point for crystalline materials
Temperature above which atomic movement is large enough to break the material’s highly ordered structure.
At temperatures higher than the Tm, material behaves as liquid and deforms via viscous flow.
Below this temperature, the substance is a highly ordered solid, with its crystal structure and grain boundaries intact.
transition temperature: amorphous ceramics
Amorphous materials do not have a distinct Tm because internal structure is not ordered and can be considered “free flowing solid”
Instead, the material becomes more and more ‘viscous’ with decreasing temperature until it can be treated as solid.
In the “solid” state, there is very little atomic movement.
transition temperature: glass transition temperature
Thermal transitions can be defined in terms of the viscosity of the material.
At lower temperatures, the material becomes more and more like a solid.
The glass transition temperature (Tg) is the temperature below which the material is considered to be a glass (solid).
overview - Tm and Tg
Tm is temp above which crystalline material is liquid (melting a metal)
Tg is temp below which amorphous material is solid (remember glass is solid at room temp)
transition temperature: polymers
polymers may behave like a liquid, a rubbery solid, or a glass depending on their temperature and molecular structure
Crystalline polymers can undergo melting at a defined Tm.
Above Tm, atoms/segments of atoms will vibrate with sufficient energy that results in motion of the overall chain
This can now break secondary bonds and order of the crystal is broken
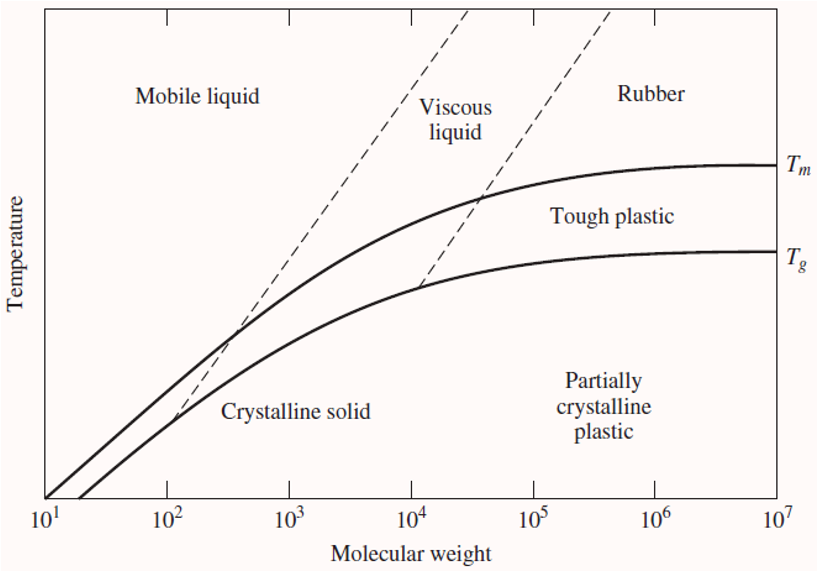
what affects melting point in crystalline polymers?
Tm occurs if crystalline phases present in polymer
Molecular weight: Tm increases with increasing molecular weight. This is because with higher molecular weight, there are fewer polymer chain ends. (More bonds need to be broken compared to lower molecular weight, more energy required)
Branching: decrease in Tm with increasing amounts of branching. more chain branching, the molecules are less densely packed and therefore cannot form van der Waals interactions or hydrogen bonds as easily. (More branching means there are less secondary bonds to break)
amorphous polymers
polymers that are amorphous possess a glass transition point similar to that of amorphous ceramics
Tg generally occurs at lower temperatures than Tm.
Below the Tg, the polymeric material is glassy and brittle, while above it, the chains are mobile enough to produce a rubbery, elastic material.
what does it mean to be rubbery?
bonds that don’t break but can move
what factors affect glass transition temperature in amorphous polymers?
Factors that influence chain vibration and rotation have a large effect on the Tg of a polymer.
Chain flexibility is one of the major determinants of the glass transition point, since more flexibility results in a greater possibility for molecular motion.
A polymer with more flexible chains will require less energy to achieve the required movement around the backbone and its Tg will be lower.
chain flexibility
Chemical constituents have the largest effect on chain flexibility.
C–O bonds rotate more easily than C–C bonds, more rotation less Tg
Addition of bulky side groups reduces movement around the backbone and increases Tg (example of bulky side group: alcohols, amines, carboxylic acids, ketones, and ethers)
Polar side groups that promote chain interactions (increased intermolecular forces, inter chain attraction and cohesion which leads to decreased free volume) also increase Tg (example: amino acids)
A C-O bond generally has more rotational freedom than a C-C bond because oxygen, being more electronegative, creates a more polar bond with carbon, leading to less significant orbital overlap and a lower energy barrier to rotation compared to the relatively non-polar C-C bond
Polar side chains contain groups that are either charged at physiological pH or groups that are able to participate in hydrogen bonding, e.g. aspartic acid, glutamine.
what happens when a polymer has both crystalline and amorphous?
Semicrystalline polymers that contain both crystalline and amorphous regions can have both glass transition and melting points.
Tm may be undetectable for polymers with low percent crystallinity.
transition temperature: crystallization temperature
For polymers with low crystallinity, possessing the ability to crystallize, at a characteristic temperature above Tg known as the crystallization temperature Tc
At this temperature, the polymer chains will have sufficient energy to move into a highly ordered crystalline state.
This arrangement of the polymer chains into a crystalline state is an exothermic process.
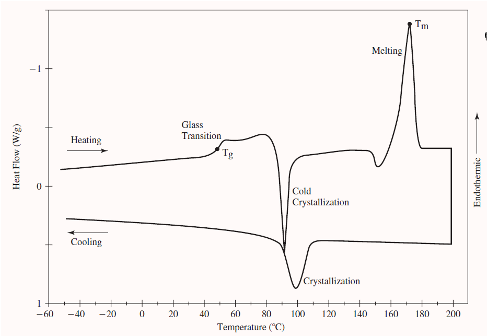
characterization methods for thermal transition
In a controlled environment, a material’s response to change in temperature can also provide useful information about the chemical or physical makeup of the material.
Thermal analysis involves the measurement of the physical properties of a material (and/or reaction products) as a function of temperature as the sample is subjected to controlled changes in temperature.
differential scanning calorimetry (DSC)
the difference in heat flow into a sample and reference material is recorded as a function of temperature while the two are exposed to a controlled temperature ramp.
There are two types of DSC experiments: power-compensated DSC and heat-flux DSC.
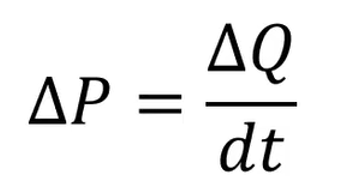
power compensated DSC
The sample and reference cells are heated by individual heaters and the temperature difference between the cells is kept near zero.
The power needed to maintain this equal temperature is then compared and this gives an idea of heat flow
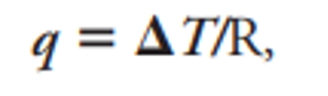
heat compensated DSC
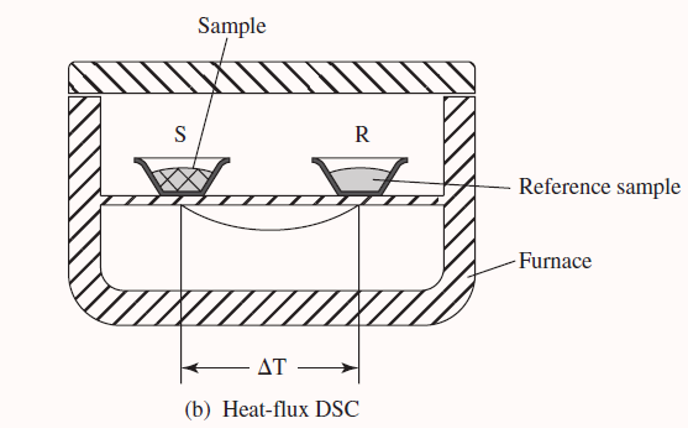
In heat-flux DSC, the sample and reference are heated from the same heater and the temperature difference between the two cells is measured.
The temperature difference is then converted to heat flow.
Q is sample heat flow and delta t is temperature difference and r is resistance of sample holder
information provided by DSC
especially useful in determining percent crystallinity of polymers.
Since the Tm is affected by the amount of crystalline material present, comparing the area under the curve representing Tm for a semicrystalline polymer with that for the same polymer in its crystalline form can provide an accurate measure of the percent crystallinity of the semicrystalline polymer